
Compliance Monitor (9/13/2024)
CHAP is always seeking resources and insights to enhance the knowledge of partners and customers.
Be sure to download CHAP’s compliance calendars for home health and hospice.
Top Items:
CMS Issues Request for Information on Potential Consolidation of Some Medicare Administrative Contractor Jurisdictions
September 4: CMS issued a Request for Information to obtain feedback from industry and the public about the potential consolidation of four Medicare Administrative Contractor (MAC) jurisdictions into two jurisdictions, as well as to obtain input on extending MAC contracts to 10 years. MACs are private health care insurers that have been awarded a geographic jurisdiction to process Medicare Part A and Part B medical claims or Durable Medical Equipment claims for people with Traditional Medicare. Information on the role of MACs can be found here .
CMS Posts FAQs Addressing BD BACTEC™ Blood Culture Bottle Shortage
August 29: As part of its responsibility to ensure quality in clinical laboratory testing, CMS posted FAQs to address a supply issue disrupting the availability of some blood culture media bottles, specifically, related to the BD BACTEC™ Blood Culture Bottle Shortage. The FAQs address the three main stakeholder concerns regarding the blood culture shortage, including CMS awareness, the use of expired blood culture bottles, and the verification of glass blood culture bottles. Additional information can be found in the statement issued by HHS Secretary Xavier Becerra here.
COVID-19 Updates
COVID-19: Updated Vaccines for 2024–2025 Season
The FDA approved updated COVID-19 vaccines for the 2024–2025 season:
- mRNA vaccines made by Pfizer-BioNTech and Moderna on August 22, 2024
- Novavax COVID-19 Vaccine, Adjuvanted on August 30, 2024
These vaccines target currently circulating variants and provide better protection against serious consequences of COVID-19, including hospitalization and death:
- Pfizer-BioNTech and Moderna COVID-19 vaccines include a monovalent (single) component that corresponds to the Omicron variant KP.2 strain of SARS-CoV-2
- Novavax COVID-19 Vaccine, Adjuvanted includes a monovalent (single) component that corresponds to the Omicron variant JN.1 strain of SARS-CoV-2
CMS updated COVID-19 vaccine pricing for the 2024–2025 season:
- CPT codes 91318–91322 effective August 22, 2024 (Note: no change to payment allowances from last season for 91318–91319)
- CPT code 91304 effective August 30, 2024
Use CPT code 90480 to bill for the administration of the vaccine.
Visit the COVID-19 Vaccine Provider Toolkit for more information. Note: You may need to refresh your browser if you recently visited this webpage.
CDC COVID-19 Wastewater Surveillance
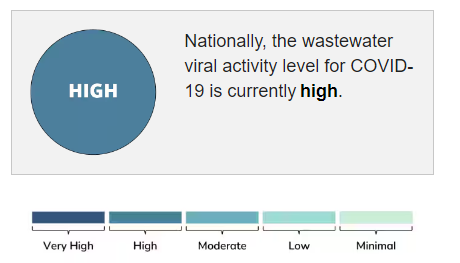
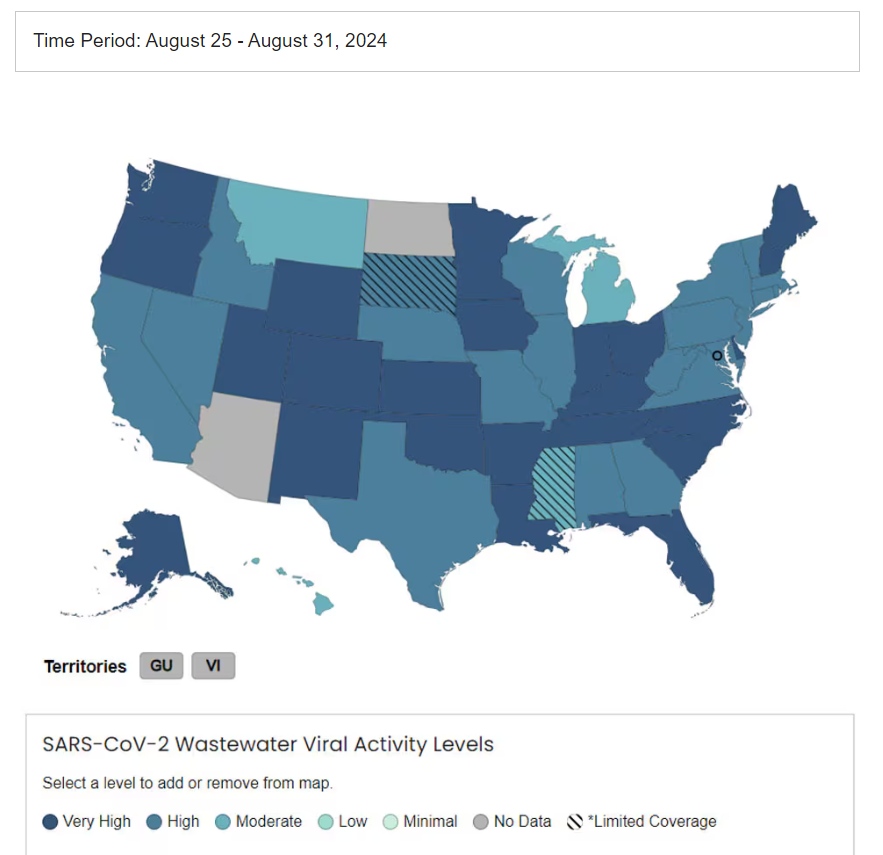
Hospice Updates
Hospice Benefit: Expanding Prepayment Review in 4 States
To combat fraud, waste, and abuse under the hospice benefit, CMS will expand prepayment medical review this September in Arizona, California, Nevada, and Texas. To help reduce burden on compliant providers, initial review volumes will be low and adjusted based on results.
If you’re noncompliant, we may implement extended review or take additional administrative actions.
Visit Hospice for coverage information.
HOPE Data Collection Timepoints Explainer Video
The Centers for Medicare & Medicaid Services is offering a 4-minute, animated explainer video for hospice providers. This video describes Hospice Outcomes and Patient Evaluations (HOPE), the timepoints, their data collection timeframes, and introduces the symptom follow-up visit (SFV), if triggered during a HOPE timepoint. HOPE data collection will be required for the Hospice Quality Reporting Program (HQRP) beginning on October 1, 2025 (FY 2026).
Watch the video
Home Health Updates
UPDATED RESOURCE AVAILABLE: From Data Elements to Quality Measures Cross-Setting Web-Based Training
The Centers for Medicare & Medicaid Services (CMS) is offering a web-based training course that provides a high-level overview of how data elements within CMS patient/resident assessment instruments are used to construct quality measures (QMs) across post-acute care (PAC) settings. The course also discusses the relationship between these data elements and Quality Reporting Program (QRP) QMs, QM calculation, and how to access reports and use QM data for quality improvement. To access the training, click on the following link: https://pac.training/courses/data_elements_2024/#/
If you have questions about accessing resources or feedback regarding trainings, please email the PAC Training Mailbox. Content-related questions should be submitted to the HH QRP Help Desk
DME Updates
Osteogenesis Stimulators: Prior Authorization Requirements Suspended
There may be confusion over whether some noninvasive osteogenesis stimulators comply with the DME 3-year expected life requirement. Effective August 28, 2024, CMS suspended prior authorization requirements for HCPCS codes E0747, E0748, and E0760.
We’ll provide additional direction about this requirement in future notice and comment rulemaking.
More Information:
- DME Center webpage: Read the full notice
- Prior Authorization and Pre-Claim Review Initiatives webpage: See recent updates
DMEPOS: Provider Level Adjustment Codes on Remittance Advice
Learn about system changes to report DMEPOS provider level adjustment codes on the remittance advice. See the instruction to your Medicare Administrative Contractor (PDF).
All Provider Updates
Organizational Providers: Do You Need to Revalidate Your Enrollment Record Soon?
Use the Medicare Revalidation List to find out if you need to revalidate your enrollment record. CMS usually posts revalidation due dates 6–7 months in advance, but we’ll establish your date at least 90 days in advance. A due date of “TBD” means that we haven’t set your due date, and you don’t need to do anything now.
We won’t issue new revalidation due dates for November 2024 – April 2025 and will resume in May 2025.
See Revalidations (Renewing Your Enrollment) for more information.
National Correct Coding Initiative: October Update
Get the National Correct Coding Initiative (NCCI) fourth quarter edit files, effective October 1, 2024, on these Medicare NCCI webpages:
Heat Injury and Illness Prevention in Outdoor and Indoor Work Settings Rulemaking
On August 30, 2024, OSHA published in the Federal Register a Notice of Proposed Rulemaking (NPRM) for Heat Injury and Illness Prevention in Outdoor and Indoor Work Settings. This is a significant step toward a federal heat standard to protect workers. The proposed standard would apply to all employers conducting outdoor and indoor work in all general industry, construction, maritime, and agriculture sectors where OSHA has jurisdiction. The standard would require employers to create a plan to evaluate and control heat hazards in their workplace. It would clarify employer obligations and the steps necessary to effectively protect employees from hazardous heat. The ultimate goal is to prevent and reduce the number of occupational injuries, illnesses, and fatalities caused by exposure to hazardous heat.
OSHA encourages the public to participate by submitting comments. The NPRM is available on the Federal Register web page at https://federalregister.gov/d/2024-14824 and at www.regulations.gov, which is the Federal e-Rulemaking Portal. You may submit comments and attachments electronically at www.regulations.gov, Docket No. OSHA-2021-0009. Follow the instructions online for making electronic submissions. Comments must be submitted by December 30, 2024.
Claim Status Category & Claim Status Codes
Learn about claims status category and code updates effective July 1, 2024:
- Accredited Standards Committee (ASC) X12 code lists, including added, changed, or deleted codes
- Examples of the ASC X12 276 and ASC X12 277 request and response transactions
New Waived Tests
Learn about billing for Clinical Laboratory Improvement Amendments waived laboratory tests (PDF):
- Requirements
- New test approved by the FDA
- Modifier QW
Medicare Administrative Contractors will adjust claims you bring to their attention.
OIG: Audit of Medicare Claim Lines for Which Payments Exceeded Charges
This is a recently added item to the OIG work plan
https://oig.hhs.gov/reports-and-publications/workplan/summary/wp-summary-0000881.asp
NASEM Workshop: Advancing Equity in Diagnostic Excellence to Reduce Health Disparities
The workshop will explore current opportunities for improving equitable diagnosis within the U.S. healthcare system, with emphasis on populations at greatest risk of harm from diagnostic errors.
- Day 1: Monday, September 23, 8:30am – 4pm ET
- Day 2: Tuesday, September 24, 8:30am – 12:30pm ET
Learn more and register for the workshop.
2024 Home Health and Hospice MAC Collaborative Summit
Registration for the 2024 Home Health and Hospice MAC Collaborative Summit is open. The theme is Perfecting Performance by Breaking Down Barriers and will be hosted in Las Vegas, NV from October 2 to 4. National Government Services, Inc. (NGS), Palmetto GBA, and CGS Administrators have designed this unique collaborative educational opportunity for home health and hospice providers from every state and Medicare jurisdiction.