7/22/22 – Weekly Covid-19 Update
CHAP’s COVID-19 Weekly Update
In this “one-stop-shop” update, CHAP will provide a roundup of important COVID-19 information from various federal sources.
All health care providers should be monitoring COVID-19 incidence rates in their state/county on an ongoing basis. The Centers for Disease Control and Prevention CDC provides weekly data about case rates, deaths, testing, and vaccine administration on their COVID tracker webpage.
CDC Data
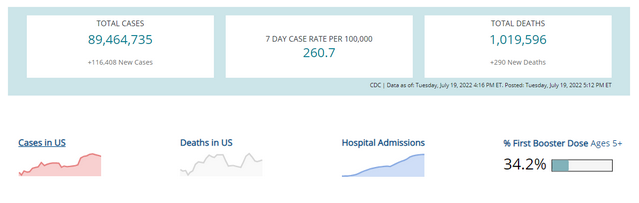
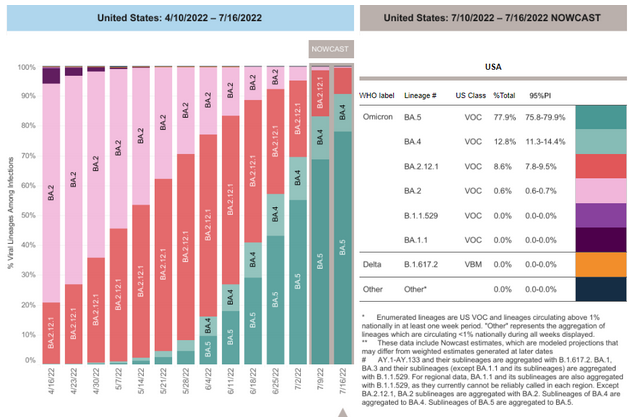
COVID-19 Variants by region
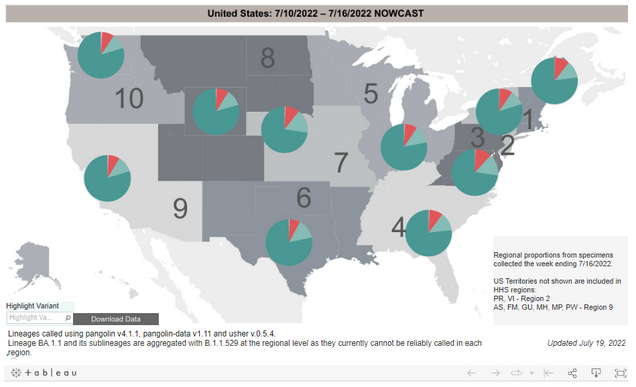
Variant Types – Map key
CDC and other Federal Updates:
COVID-19 Public Health Emergency (PHE) Extended
The current COVID-19 PHE declaration was extended another 90 days and will expire on 10/13/22. All CMS waivers remain in place.
FDA Authorizes Emergency Use of Novavax COVID-19 Vaccine, Adjuvanted (FDA, 7/13/22)
The FDA issued an emergency use authorization (EUA) for the Novavax COVID-19 Vaccine, Adjuvanted for the prevention of COVID-19 caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in individuals 18 years of age and older. The Novavax COVID-19 Vaccine, Adjuvanted is administered as a two-dose primary series, three weeks apart. The vaccine contains the SARS-CoV-2 spike protein and Matrix-M adjuvant. Adjuvants are incorporated into some vaccines to enhance the immune response of the vaccinated individual. The authorization offers adults in the United States who have not yet received a COVID-19 vaccine another option
SARS-CoV-2 Viral Mutations: Impact on COVID-19 Tests (FDA, 7/14/22)
This webpage provides information about tests authorized by the U.S. Food and Drug Administration (FDA) for the identification and differentiation of specific SARS-CoV-2 mutations and lineages as well as the impact of viral mutations on COVID-19 tests.
FDA Updates (7/19/22)
- The FDA updated the device shortage list and the device discontinuance list on the web page for Medical Device Shortages During the COVID-19 Public Health Emergency. In addition, the FDA is providing information about devices that have been removed from the device shortage list at this time.
- The FDA updated its Supplies of Medical Devices for COVID-19: Frequently Asked Questions web page to provide information about how the FDA determines to remove product codes from the device shortage list and what the FDA is doing to help medical device manufacturers with the global shortage of semiconductor chips.