6/24/22 – Weekly Covid-19 Update
CHAP’s COVID-19 Weekly Update
In this “one-stop-shop” update, CHAP will provide a roundup of important COVID-19 information from various federal sources.
All health care providers should be monitoring COVID-19 incidence rates in their state/county on an ongoing basis. The Centers for Disease Control and Prevention CDC provides weekly data about case rates, deaths, testing, and vaccine administration on their COVID tracker webpage.
CDC Data
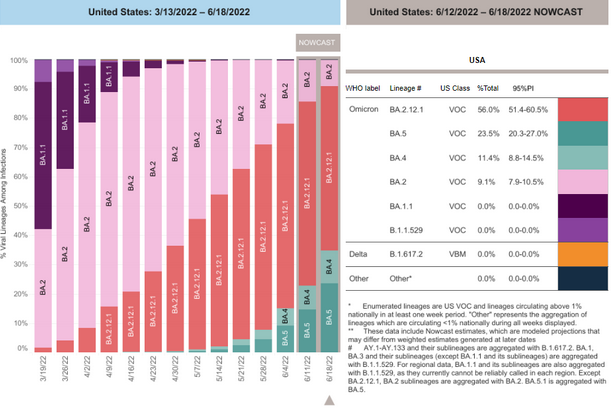
COVID-19 Variants by region
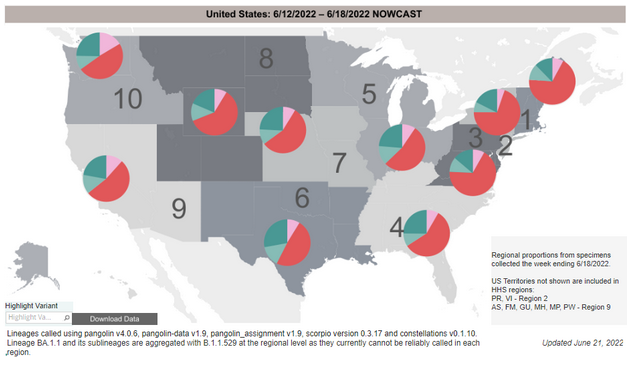
Variant Types – Map key
CDC and other Federal Updates:
FDA Authorizes Vaccines for Young Children (FDA, 6/17/22)
The FDA authorized emergency use of the Moderna COVID-19 Vaccine and the Pfizer-BioNTech COVID-19 Vaccine for the prevention of COVID-19 to include use in children down to 6 months of age.
For the Moderna COVID-19 Vaccine, the FDA amended the emergency use authorization (EUA) to include use of the vaccine in individuals 6 months through 17 years of age. The vaccine had been authorized for use in adults 18 years of age and older.
The Moderna COVID-19 Vaccine is administered as a primary series of two doses, one month apart, to individuals 6 months through 17 years of age. The vaccine is also authorized to provide a third primary series dose at least one month following the second dose for individuals in this age group who have been determined to have certain kinds of immunocompromise.
For the Pfizer-BioNTech COVID-19 Vaccine, the FDA amended the EUA to include use of the vaccine in individuals 6 months through 4 years of age. The vaccine had been authorized for use in individuals 5 years of age and older.
The Pfizer-BioNTech COVID-19 Vaccine is administered as a primary series of three doses in which the initial two doses are administered three weeks apart followed by a third dose administered at least eight weeks after the second dose in individuals 6 months through 4 years of age.
Fact Sheet: What happens to premiums if the extra help from the American Rescue Plan expires? (HHS, 6/22/22)
On March 11, 2021, President Joe Biden signed a new law (American Rescue Plan) to combat COVID-19 and build our country back better. The American Rescue Plan included subsidies that lower health care costs and expand access to affordable, comprehensive health coverage.
If Congress does not extend the ARP, HHS analysis projects that:
- Many Health Insurance Marketplace consumers across the country – in rural and urban areas – will likely see substantial increases in out-of-pocket premium costs
- The number of uninsured Americans will increase significantly
- Approximately 3 million Americans could lose their health insurance
- More than 10 million Americans will have reduced premium tax credits or lose them entirely:
- 8.9 million people will have their tax credits reduced (averaging $406 per person, annually)
- 1.5 million people will lose subsidies entirely (averaging $3,277 per person, annually)