
Proposed CY 2026 HH PPS Payment Update Rule Posted
The proposed Calendar Year 2026 Home Health Prospective Payment System (HH PPS) Rate Update; Requirements for the HH Quality Reporting Program and the HH Value-Based Purchasing Expanded Model; Durable Medical Equipment, Prosthetics, Orthotics, and Supplies (DMEPOS) Competitive Bidding Program Updates; DMEPOS Accreditation Requirements; Provider Enrollment; and Other Medicare and Medicaid Policies was posted on the Federal Register Public Inspection desk on 6/30/2025.
Providers are strongly encouraged to review the rule in its entirety as this is only a summary of the content and to send comments to CMS by August 29, 2025. Here are the highlights of the final rule.
Please note: The Provider Enrollment, Certain Durable Medical Equipment, Prosthetics, Orthotics, and Supplies (DMEPOS) Accreditation Policies, and DMEPOS Prior Authorization content in this proposed rule appears in a separate document. While the subject matter is part of the rule, CHAP carved it out into a separate document to aid DMEPOS provider review.
Payment Update Information
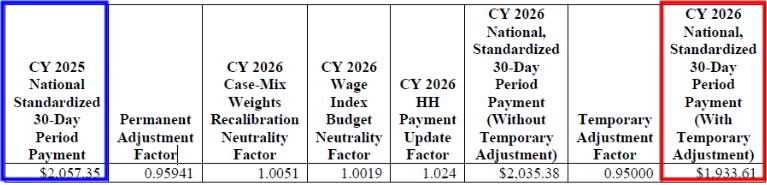
CMS is proposing a rate cut for the fourth straight year of rulemaking. CMS estimates that Medicare payments to HHAs in CY 2026 would decrease in the aggregate by 6.4%, or $1.135 billion, compared to CY 2025, based on the proposed policies. See the breakout below.
- Proposed CY 2026 rates include a 2.4% HH payment increase ($425M)
- A 3.7% decrease due to permanent behavior adjustment ($655M)
- A 4.6% decrease from the temporary adjustment ($815M)
- A 0.5% decrease from FDL ratio update ($90M)
CY 2026 National, Standardized 30-Day Period Payment Amount (Figure appears on page 103 of the rule)
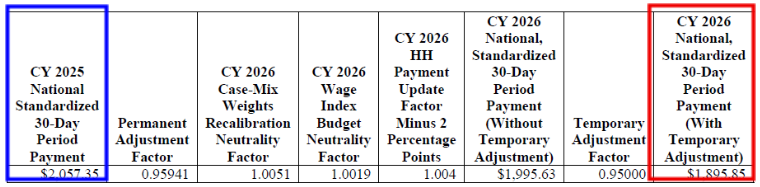
CY 2026 National, Standardized 30-Day Period Payment Amount for HHAs That Do Not Submit the Quality Data (Figure appears on page 103 of the rule)
The CY 2026 national standardized 30-day period payment rate for an HHA that does not submit the required quality data would be updated by 0.4 percent (the proposed CY 2026 home health payment update percentage of 2.4 percent minus 2 percentage points)
PDGM and Behavior Assumptions
Statute requires CMS to annually determine the impact of differences between assumed behavior changes and actual behavior changes on estimated aggregate expenditures, beginning with 2020 and ending with 2026, and to make temporary and permanent increases or decreases, as needed, to the 30-day payment amount to offset such increases or decreases. The temporary adjustment has been accruing at approximately $1 billion per year since CY 2020, we believe beginning to adjust the base payment rate now to account for the calculated temporary dollar amount to date may help reduce the need for a larger reduction in future years.
For the CY 2026 HH PPS proposed rule, CMS used CY 2024 claims and determined Medicare still paying more under the updated system than the old. Thus, we propose a permanent adjustment of -4.059% to the 30-day base payment rate. This proposal meets statutory requirements to balance expenditures, reduces the need for future large adjustments, and helps slow temporary payment accruals. The adjustment also aims to minimize potential temporary adjustments in future years.
- CMS is proposing to introduce a temporary 5.0% reduction in the national, standardized payment rate for the calendar year (CY) 2026. This proposal aims to initiate the recoupment of retrospective overpayments made during CYs 2020 through 2024
- They anticipate making additional temporary adjustments for CY 2025 and 2026, once data for those years are available.
- Not included – the -5.0% temporary adjustment applied for CY 2026 when calculating the CY 2027 base payment rates, as the law requires us to analyze the claims data each year through CY 2026
Opportunity to Comment: CMS is soliciting comments on the proposals to apply the permanent adjustment of -4.059 percent and the -5.0 percent temporary adjustment to the CY 2026 home health base payment rate.
Proposed CY 2026 PDGM LUPA Thresholds
- CMS proposes to update the LUPA thresholds using CY 2024 home health claims utilization data (as of March 13, 2025), in accordance with our policy to annually recalibrate the case-mix weights and update the LUPA thresholds, functional impairment levels, and comorbidity subgroups
- The proposed LUPA thresholds for the CY 2026 PDGM payment groups with the corresponding Health Insurance Prospective Payment System (HIPPS) codes and the case-mix weights are listed in Table 25 on pages 78 – 90 of the rule.
Opportunity to Comment: CMS is soliciting public comments on the proposed updates to the LUPA thresholds for CY 2026. The proposed LUPA thresholds will be updated based on more complete CY 2024 claims data in the final rule.
Recalibration of PDGM Case-Mix Weights and comorbidity subgroups
- Changes to the PDGM case-mix weights are implemented in a budget neutral manner by multiplying the CY 2026 national standardized 30-day period payment rate by a case-mix budget neutrality factor.
- The case-mix budget neutrality factor for CY 2026 of 1.0051
- CMS is proposing to use CY 2024 claims data to update the functional points and functional impairment levels by clinical group using the same methodology previously finalized to update the functional impairment levels for CY 2026
- The proposed updated OASIS functional points table and the table of functional impairment levels by clinical group for CY 2026 are listed in tables 20 and 21, on pages 60-62 of the rule
- The proposed case-mix weights for CY 2026 are listed in Table 25 and will also be posted
- on the HHA Center webpage upon display of this proposed rule
- CMS proposes to update the comorbidity subgroups to include 20 low comorbidity adjustment subgroups and 100 high comorbidity adjustment interaction subgroups
- The proposed CY 2026 low comorbidity adjustment subgroups and the high comorbidity adjustment interaction subgroups including those diagnoses within each of these comorbidity adjustments are shown in tables 22 and 23 on pages 63-71 of the rule.
Opportunity to Comment: CMS is soliciting public comments on the proposed updates to functional points and the functional impairment levels by clinical group, the low comorbidity adjustment subgroups and the high comorbidity adjustment interactions for CY 2026, and the CY 2026 proposed case-mix weights and proposed case-mix weight budget neutrality factor.
Proposed CY 2026 National Per-Visit Rates for 30-day Periods of Care
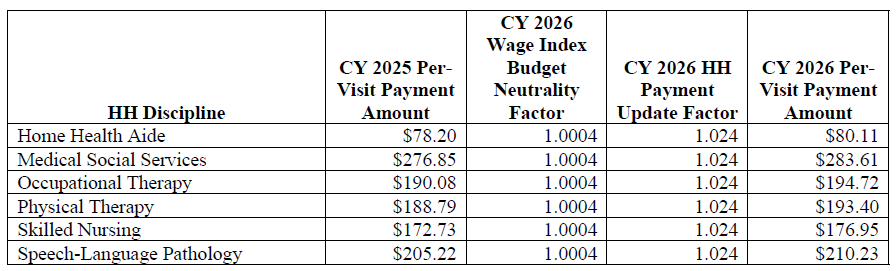
- The national per-visit rates are used to pay LUPAs and are also used to compute imputed costs in outlier calculations (See table 28 on page 105 of the rule)
- The proposed CY 2026 national per-visit rates for HHAs that submit the required quality data are updated by the proposed CY 2026 home health payment update percentage of 2.4 percent
CY 2026 National Per-Visit Payment Amounts
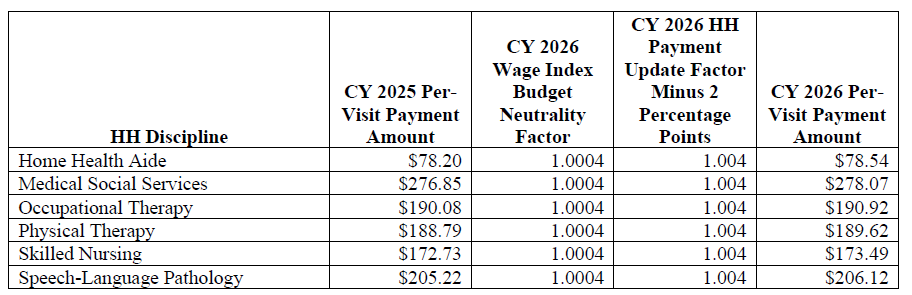
- CY 2026 per-visit payment rates for HHAs that do not submit the required quality data would be updated by 0.4 percent, which is the proposed CY 2026 home health payment update percentage of 2.4 percent minus 2 percentage points (See table 28 on page 105 of the rule)
CY 2026 National Per-Visit Payment Amounts for HHAs That Do Not Submit the Required Quality Data
Opportunity to Comment: CMS is soliciting comments on the proposed CY 2026 30-day home health payment rates and the per-visit payment rates.
Proposed Home Health Regulatory Changes
CMS proposes to:
- revise § 424.22(a)(1)(v)(A) to state that the face-to-face encounter must be performed by one of the following: a physician, a nurse practitioner, a clinical nurse specialist, or a physician assistant as defined at 42 CFR 484.2; or a certified nurse-midwife as defined in section 1861(gg)) of the Act as authorized by State law
- remove § 424.22(a)(1)(v)(C), which limits the face-to-face encounter to the certifying physician or allowed practitioner unless the encounter is performed by either of the following:
- A certified nurse midwife as described in paragraph (a)(1)(v)(A)(4) of this section.
- A physician, physician assistant, nurse practitioner, or clinical nurse specialist with privileges who cared for the patient in the acute or post-acute facility from which the patient was directly admitted for home health care and who is different from the certifying practitioner
- remove at § 484.45(a) to remove the term “beneficiary” and replace it with the term “patient”
Opportunity to Comment: CMS is soliciting comments on proposed revisions to 424.22(a)(1)(v).
Home Health Quality Reporting Program (QRP)
Removal of HHQRP data
CMS proposes to remove the “COVID-19 Vaccine: Percent of Patients Who Are Up to Date” measure and the item related to the measure and corresponding data element and removal of four SDOH assessment items: one Living Situation item, two Food items, and one Utilities item.
CMS proposes that the Patient/Resident COVID-19 measure rates would be publicly reported for the last time with the January 2026 Care Compare refresh on Medicare.gov based on data from Q1 of 2025.
Opportunity to Comment: CMS is soliciting comments on proposed end the public display of Patient/Resident COVID-19 Measure data after the January 2026 Care Compare refresh.
Reconsideration policy
CMS proposes to revise the Reconsideration policy to allow providers that fail to provide complete, timely data to CMS to submit a request for reconsideration if they can demonstrate full compliance
- In very limited circumstances, CMS would permit the HHA to request an extension to file a reconsideration request if the HHA was affected by an extraordinary circumstance beyond the control of the HHA (that is, a natural disaster such as a hurricane tornado or earthquake) during the 30-day reconsideration period and codify this process at § 484.245(d)(6)
- CMS would notify the HHA in writing of its final decision regarding its request for an extension to file a reconsideration of non-compliance request via an email from CMS
Update to all-payer data submission of OASIS
CMS proposes to update the Home Health Agency CoPs to account for all-payer data submission of OASIS data
- To align with the transition to OASIS all-payer submission requirements as outlined in the CY 2023 Home Health PPS final rule, CMS proposes at § 484.45(a) to remove the term “beneficiary” and replace it with the term “patient”
- § 484.45(a) would read, if finalized, “An HHA must encode and electronically transmit each completed OASIS assessment to the CMS system, regarding each patient with respect to which information is required to be transmitted (as determined by the Secretary), within 30 days of completing the assessment of the patient”
Revised HHCAHPS Survey
CMS proposes implementing a revised HHCAHPS Survey beginning with the April 2026 sample month.
- No survey administration changes are proposed with the new survey
- The revised survey is shorter than the current survey
- It includes changes to the survey and the quality measures derived from testing include the following:
- Addition of three new questions to assess new topics of importance to patients
- ++ Whether the care provided helped the patient take care of their health
- ++ Whether the patient’s family/friends were given sufficient information and instructions
- Removal of questions or topics of less importance to patients (that is, six questions about medications were reduced to two questions).
- Removal of questions not currently used in public reporting composites (that is, three questions on which type of staff served the patient—nurse, physical or occupational therapist, and home care aide).
- Removal of one question which did not perform well in testing to stand alone or fit into one of the revised composite measures:
- Whether the patient got information about what care and services they would get when they first started getting home health care
- Minor text changes to selected existing questions to help clarify the question or response options, based on feedback from patients
- See TABLE 31: Current And Proposed Changes to HHCAHPS Survey Measures for a current and proposed survey comparison (pages 133-134)
- Addition of three new questions to assess new topics of importance to patients
- Impact of survey changes on Star Ratings:
- The revised Care of Patients measure is conceptually like the current Care of Patients measure, but the change (adding two new questions and dropping one question) is substantive and the revised measure should be treated as new
- The revised Communications Between Providers and Patients measure is similar to the current Communications Between Providers and Patients measure but the change (dropping two questions and adding one new question) is substantive and the revised
- measure should be treated as new
- CMS anticipates the first Care Compare refresh in which publicly reported measures scores would be updated to include the new measures would be October 2027, with scores calculated using data from Q2 2026 through Q1 2027
- During the transition period, scores and Star Ratings for the Overall Rating and Willingness to Recommend measures would be calculated by combining scores from quarters using the current and new survey and continue to be reported
Opportunity to Comment: CMS invites public comments on the proposal to remove the COVID-19 Vaccine measure from the HH QRP beginning with the CY 2026 HH QRP, the removal of four standardized patient assessment data elements collected under the SDOH category from the HH QRP, the amendment of the HHA QRP Reconsideration policy and the HHCAHPS Survey proposals.
Policies Regarding Public Display of Measure Data for the HH QRP
CMS proposes that the Patient/Resident COVID-19 measure rates would be publicly reported for the last time with the January 2026 Care Compare refresh on Medicare.gov based on data from Q1 of 2025.
We invite public comments on our proposal to end the public display
of Patient/Resident COVID-19 Measure data after the January 2026 Care Compare refresh on
Medicare.gov.
Request for Information (RFI)
Measure Concepts Under Consideration for Future Years
CMS seeks public input on four concepts for future measures for the HH QRP which it intends to use to inform future measure development efforts.
- Interoperability – CMS defines “interoperability” in part, and with respect to health IT, as health IT that enables the secure exchange of electronic health information with, and use of electronic health information from, other health IT without requiring special efforts by the user.
- They request input and comment on approaches to assessing interoperability in the HH setting, for instance, measures that address or evaluate the level of readiness for interoperable data exchange, or measures that evaluate the ability of data systems to securely share information across the spectrum of care.
- Cognitive Function – This RFI is requesting input on cognitive functioning measures that may be available for immediate use, or that may be adapted or developed for use in the HH QRP, using the BIMS or the CAM measurement tools.
- CMS also seeks input on the feasibility of measuring:
- improvement in cognitive functioning during a HH stay, which typically averages 56 days
- the cognitive skills (example executive functions) that are more likely to improve during an HHA stay
- conditions for which measures of maintenance – rather than improvement in cognitive functioning – are more practical
- the types of intervention that have been demonstrated to assist in improving or maintaining cognitive functioning
- CMS also seeks input on the feasibility of measuring:
- Well-Being – CMS seeks input on a quality measure concept of nutrition specifically feedback on tools and frameworks that promote healthy eating habits, exercise, nutrition, or physical activity for optimal health, well-being, and best care for all.
Potential Revision of the Final Data Submission Deadline Period from 4.5 Months to 45 Days
CMS seeks feedback on a potential change to the final data submission deadline from 4.5 months to 45 days after the close of the period which they intend to use to inform program improvement efforts.
- CMS has become concerned that the time between when data are collected and when the measures are reported from those data may be too long to get the desired results in a public reporting program
- Currently, the largest contributing factor to the 9- month lag between end of the data collection and when measures are publicly reported is the current 4.5-month timeframe for data submission
- If the timeframe for data submission was reduced from 4.5 months to 45 days, the lag time between collection and reporting could be reduced by up to 3 months, which would result in more timely public reporting
- CMS requests feedback on a potential future reduction of the HH QRP data submission deadline from 4.5 months to 45 days. Specifically: following:
- How this potential change could improve the timeliness and actionability of HH QRP
- How this potential change could improve public display of quality information
- How this potential change could impact HHA workflows or require updates to Systems
Advancing Digital Quality Measurement in the HH QRP
As part of an effort to advance the digital quality measurement (dQM) transition, CMS is issuing this request for information (RFI) to gather broad public input on the dQM transition in HHAs. They seek feedback on the current state of health IT use, including electronic health records (EHRs), in HHAs. Specific questions for feedback appear on pages 144-147.
Deregulation Request for Information (RFI)
On January 31, 2025, President Trump issued Executive Order (EO) 14192 “Unleashing Prosperity Through Deregulation,” which states the Administration policy to significantly reduce the private expenditures required to comply with Federal regulations to secure America’s economic prosperity and national security and the highest possible quality of life for each citizen.
CMS would like public input on approaches and opportunities to streamline regulations and reduce administrative burdens on providers, suppliers, beneficiaries, and other stakeholders participating in the Medicare program.
Expanded Home Health Value-Based Purchasing (HHVBP) Model
Changes to the Applicable Measure Set
- Proposed changes to the HHCAHPS survey will affect the survey questions used to calculate three measures that are currently used in the expanded HHVBP Model. CMS proposes to remove the following measures:
- Care of Patients, Communications between Providers and Patients, and Specific Care Issues
- CMS proposes the following increase the number of OASIS-based measures used in the Model, allowing for more robust measurement of HHA performance:
- the addition of four measures to the applicable measure set as follows:
- Three OASIS-based measures related to bathing and dressing beginning with CY 2026:
- Improvement in Bathing (based on OASIS item M1830)
- Improvement in Upper Body Dressing (based on OASIS item M1810)
- Improvement in Lower Body Dressing (based on OASIS item M1820)
- One claims-based measure, the Medicare Spending per Beneficiary for the Post-Acute Care (PAC) setting measure
- Three OASIS-based measures related to bathing and dressing beginning with CY 2026:
- the addition of four measures to the applicable measure set as follows:
Updates to Individual Measure Weights and Category Weights
The OASIS-based, claims-based, and HHCAHPS Survey-based measures currently contribute 35% 35%, and 30%, to the Total Performance Score (TPS) for HHAs in the larger-volume cohort.
- CMS proposes to adjust the measure category weights for the larger-volume cohort such that the OASIS-based and claims-based measure categories each contribute 40%, and the HHCAHPS Survey-based measure category contributes 20% to the TPS due to the reduction in the number of individual HHCAHPS Survey-based measures.
- Proposed changes to the applicable measure set would increase the number of OASIS-based measures from three measures to six and increase the number of claims-based measures from two to three. The number of individual measures for the HHCAHPS Survey-based measures would decrease from five to two.
Opportunity to Comment: CMS invites public comments on the proposals to changes in the applicable measure set and updates to individual measure weights and category weights.
HHVBP Quality Measure Concepts Under Consideration for Future Years – Request for Information (RFI)
CMS requests feedback about the addition of a respecified Falls with Major Injury measure as well as two potential changes to the HHCAHPS survey-based measures scoring rules and applicable measure set as they relate to the expanded HHVBP Model.
Falls with Major Injury Measure (OASIS-based and Claims-based)
CMS reported that a recent study found that more than half of falls with a major injury identified using Medicare claims data) were not reported on OASIS assessments. OIG observed that a low fall rate reported on Care Compare may reflect a provider’s lack of falls reporting, rather than a low incidence of falls among its patients. OIG further observed that HHAs with low falls with major injury rates on Care Compare were more likely than other HHAs not to report falls among patients enrolled in Medicare.
CMS is currently working on a respecified version of the FMI measure that uses fee-for-service claims, encounter data, and OASIS data. Using multiple data sources will produce a more robust and complete data set, allowing the respecified FMI measure to be more accurate and include more providers.
CMS requests comments related to the potential addition of the respecified FMI measure to the measure set for the expanded HHVBP Model.
Potential Future Changes to HHCAHPS Scoring Rules and Applicable Measure Set
CMS anticipates proposing new HHCAHPS Survey-based measures to replace the Care of Patients, Communication Between Providers and Patients, and Specific Care Issues measures through future rulemaking. These revised HHCAHPS Survey-based measures will be based on data collected from the revised HHCAHPS Survey instrument.
CMS seeks public comments on the possibility of initially measuring HHA performance on the future HHCAHPS Survey-based measures based solely on achievement, rather than both achievement and improvement.
Adding to the Applicable Measure Set for the Expanded HHVBP Model the Three Remaining Items in the Specific Care Issues Measure as Single Item Measures
CMS proposes to modify the HHCAHPS Survey instrument. Among other changes, this proposal would remove several items used in the multi-item Specific Care Issues measure. Three of the items used in the Specific Care Issues measure will remain in the HHCAHPS Survey instrument. The three items from the Specific Care Issues measure included in the revised HHCAHPS Survey instrument are as follows:
- When you first started getting home health care from this agency, did someone from the agency talk about ways to help make your home safer?
- Has someone from the agency ever reviewed the prescribed and over-the-counter medicines you were taking?
- In the last 2 months of care, did home health staff from this agency talk with you about any side effects of your medicines?
CMS seeks public comments on the possibility of adding these three remaining HHCAHPS Survey items to the expanded HHVBP Model as single-item measures. They also seek public comments on the possibility of giving each of these single item measures a weight of one third the weight of the other HHCAHPS items, thus maintaining the same relative weight of the Specific Care Issues measure.
Review the proposed rule on the Federal Register Public Inspection desk
https://public-inspection.federalregister.gov/2025-12347.pdf
It is anticipated that this proposed rule will be posted in the Federal Register on/after 7/4/2025.
For further information, see the CMS summary of the HHA Final rule – https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2026-home-health-prospective-payment-system-proposed-rule-fact-sheet-cms-1828-p#_ftn1
Questions about the content of this rule? Contact CHAP