
Final CY 2026 HH PPS Payment Update Rule Posted
The final Calendar Year 2026 Home Health Prospective Payment System (HH PPS) Rate Update; Requirements for the HH Quality Reporting Program and the HH Value-Based Purchasing Expanded Model; Durable Medical Equipment, Prosthetics, Orthotics, and Supplies (DMEPOS) Competitive Bidding Program Updates; DMEPOS Accreditation Requirements; Provider Enrollment; and Other Medicare and Medicaid Policies was posted on the Federal Register Public Inspection desk on 11/28/2025. It is anticipated that this final rule will be posted in the Federal Register on/after 12/5/2025.
These regulations are effective on January 1, 2026
Providers are strongly encouraged to review the final rule in its entirety as this is only a summary of the content. Here are the highlights of the final rule.
Please note: The Provider Enrollment, Certain Durable Medical Equipment, Prosthetics, Orthotics, and Supplies (DMEPOS) Accreditation Policies, and DMEPOS Prior Authorization content in this final rule appears in a separate document. While the subject matter is part of the rule, CHAP carved it out into a separate document to aid DMEPOS provider review.
Payment Update Information
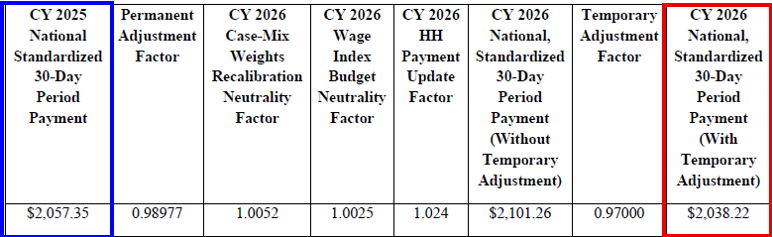
CMS is finalizing a rate cut in the aggregate by 1.3%, or $220M, compared to CY 2025, based on finalized policies. See the breakout below.
- Final national CY 2026 rates include a 2.4% HH payment increase ($405M)
- A 2.7% decrease from the temporary adjustment ($460M)
- A 0.1% decrease from FDL ratio update ($15M)
- A permanent prospective adjustment to the CY 2026 home health payment rate of -1.023%, to account for the impact of implementing the PDGM for CYs 2020 through CY 2022
CY 2026 National, Standardized 30-Day Period Payment Amount (Figure appears on page 134 of the rule)
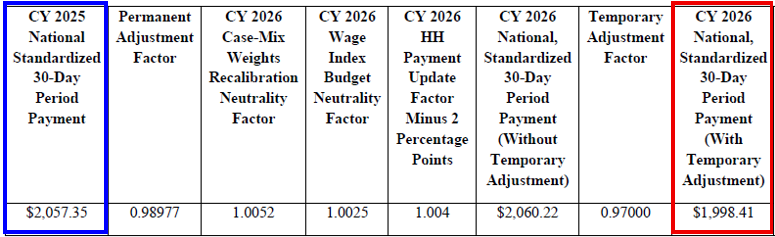
CY 2026 National, Standardized 30-Day Period Payment Amount for HHAs That Do Not Submit Quality Data (Figure appears on page 134 of the rule)
The CY 2026 national standardized 30-day period payment rate for an HHA that does not submit the required quality data would be updated by 0.4 percent (the final CY 2026 home health payment update percentage of 2.4 percent minus 2 percentage points)
PDGM and Behavior Assumptions
Statute requires CMS to annually determine the impact of differences between assumed behavior changes and actual behavior changes on estimated aggregate expenditures, beginning with 2020 and ending with 2026, and to make temporary and permanent increases or decreases, as needed, to the 30-day payment amount to offset such increases or decreases.
CMS modified the permanent adjustment from the proposed rule after commenters raised concerns that behavior change after CY 2022 might be attributable to factors unrelated to the implementation of the PDGM, such as the introduction of the OASIS-E assessment; expansion of home health value-based purchasing; and increased Medicare Advantage penetration.
This rule finalizes a permanent prospective adjustment to the CY 2026 home health payment rate of -1.023%, to account for the impact of implementing the PDGM for CYs 2020 through CY 2022. This adjustment accounts for differences between assumed behavior changes and actual behavior changes on estimated aggregate expenditures due to the CY 2020 implementation of the PDGM and the change to a 30-day unit of payment.
Final CY 2026 PDGM LUPA Thresholds
- CMS finalizes the proposal to update the LUPA thresholds for CY 2026 using CY 2024 claims data (as of July 11, 2025).
- The final LUPA thresholds for the CY2026 PDGM payment groups with the corresponding Health Insurance Prospective Payment System (HIPPS) codes and the case-mix weights are listed in table 8 (page 72) and are also available on the
- HHA Center web page, located at https://www.cms.gov/medicare/enrollment-renewal/providerssuppliers/home-health-agency-center
Recalibration of PDGM Case-Mix Weights and comorbidity subgroups
- Changes to the PDGM case-mix weights are implemented in a budget neutral manner by multiplying the CY 2026 national standardized 30-day period payment rate by a case-mix budget neutrality factor.
- The case-mix budget neutrality factor for CY 2026 of 1.0051
- CMS is finalizing the functional points and functional impairment level updates for CY 2026 as proposed, using updated CY 2024 claims data (as of July 11, 2025).
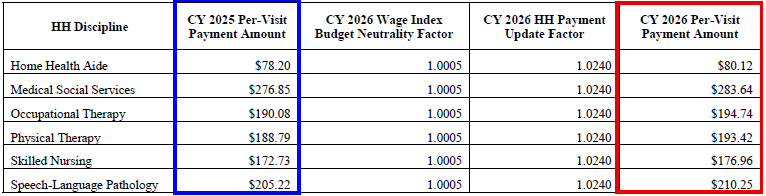
CY 2026 National Per-Visit Rates for 30-day Periods of Care
- The national per-visit rates are used to pay LUPAs and are also used to compute attributed costs in outlier calculations.
- The per-visit rates are paid by type of visit or home health discipline are updated by the final CY 2026 home health payment update percentage of 2.4 percent
CY 2026 National Per-Visit Payment Amounts
CY 2026 National Per-Visit Payment Amounts for HHAs That Do Not Submit the Required Quality Data
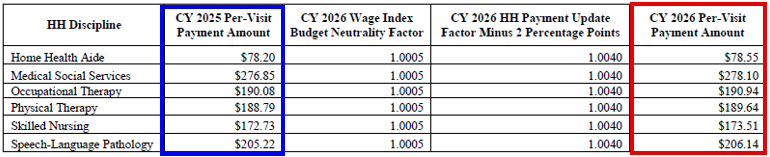
- CY 2026 per-visit payment rates for HHAs that do not submit the required quality data would be updated by 0.4 percent, which is the final CY 2026 home health payment update percentage of 2.4 percent minus 2 percentage points.
Final Home Health Regulatory Changes
As a condition for payment, prior to certifying a patient’s eligibility for the home health benefit, the physician must document that the physician himself or herself or a non-physician practitioner (NPP) has had a face-to-face encounter with the patient
CMS finalized the face-to-face requirement as follows:
- Revise § 424.22(a)(1)(v)(A) to state that the face-to-face encounter must be performed by one of the following: a physician, a nurse practitioner, a clinical nurse specialist, or a physician assistant as defined at 42 CFR 484.2; or a certified nurse-midwife as defined in section 1861(gg)) of the Act as authorized by State law
- Remove § 424.22(a)(1)(v)(C), which limits the face-to-face encounter to the certifying physician or allowed practitioner unless the encounter is performed by either of the following:
- A certified nurse midwife as described in paragraph (a)(1)(v)(A)(4) of this section.
- A physician, physician assistant, nurse practitioner, or clinical nurse specialist with privileges who cared for the patient in the acute or post-acute facility from which the patient was directly admitted for home health care and who is different from the certifying practitioner
- Remove the term “beneficiary” at § 484.45(a) and replace it with the term “patient”
CMS notes that these changes provide additional flexibility by allowing more providers to conduct the face-to-face encounter.
The amended regulatory text will appear as follows:
§ 484.45 [Amended]
29. Section 484.45 is amended in paragraph (a) by removing the word “beneficiary” and
adding in its place the word “patient” each time it appears.
30. Section 484.55 is amended by revising paragraph (d)(1)(i) to read as follows:
Home Health Quality Reporting Program (QRP)
The 19 measures currently adopted for the cy 2026 HH QRP are as follows:
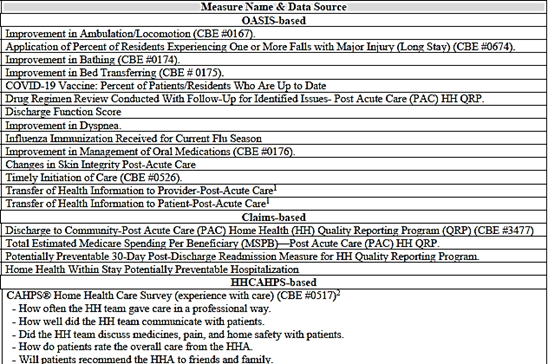
Removal of HH QRP data
CMS finalized removal the “COVID-19 Vaccine: Percent of Patients Who Are Up to Date” measure and the item related to the measure and corresponding data element and removal of four SDOH assessment items: one Living Situation item, two Food items, and one Utilities item.
Beginning with patients discharged on or after April 1, 2026, HHAs would not be required to collect and submit the Patient/Resident COVID-19 Vaccine measure data to CMS. CMS will cease publicly reporting data for this measure after the January 2026 Care Compare refresh.
- Until that time and with the posting of this final rule, HHAs may submit any valid response (0 – No, 1-Yes or dash) on a Transfer, Death at home, or Discharge OASIS assessment, without any future quality measure implications.
Removal of Four Standardized Patient Assessment Data Elements Beginning with the CY 2026 HH QRP
CMS finalized removal of four standardized patient assessment data elements (one item for Living Situation (R0310); two items for Food (R0320A and R0320B); and one item for Utilities (R0330)) collected under the SDOH category from the HH QRP beginning with the CY 2026 HH QRP without modification. They state that because data collection has not begun and they do not have an active use for these items, they re-evaluated the value of adding them to the OASIS at this time.
Reconsideration Request policy/Codifying the Bases on Which CMS Can Grant a Reconsideration Request
CMS established a process for HHAs to request exceptions and extensions for HH QRP reporting requirements starting with CY 2019 when extraordinary circumstances occur beyond their control. If granted, the HHA’s PPS payment will not be reduced for noncompliance. CMS also allows, in limited cases, an HHA to extend the deadline for reconsideration requests.
CMS finalized revision of the Reconsideration policy to allow providers that fail to provide complete, timely data to CMS to submit a request for reconsideration if they can demonstrate full compliance
- In very limited circumstances, CMS would permit the HHA to request an extension to file a reconsideration request if the HHA was affected by an extraordinary circumstance beyond the control of the HHA (that is, a natural disaster such as a hurricane tornado or earthquake) during the 30-day reconsideration period and codify this process at § 484.245(d)(6)
- CMS would notify the HHA in writing of its final decision regarding its request for an extension to file a reconsideration of non-compliance request via an email from CMS
CMS finalized the proposal to amend the bases by which they grant a reconsideration request under the HH QRP reconsideration policy and to codify this policy at § 484.245(d)(5).
The updated regulatory text will appear as follows:
§ 484.245 Requirements under the Home Health Quality Reporting Program (HH QRP).
* * * * *
(d) * * * *
(4)(i) CMS notifies the HHA, in writing, of its final decision regarding any
reconsideration request through at least one of the following methods:
(A) CMS designated data submission system.
(B) The United States Postal Service.
(C) Email from the CMS Medicare Administrative Contractor (MAC).
(ii) CMS grants a timely request for reconsideration, and reverses an initial finding of
non-compliance, only if CMS determines that the HHA was in full compliance with the HH QRP requirements for the applicable program year.
(5)(i) An HHA may request, and CMS may grant, an extension to file a reconsideration
request if, during the period to request a reconsideration as set forth in paragraph (d)(2) of this
section, the HHA was affected by an extraordinary circumstance beyond the control of the HHA
(for example, a natural or man-made disaster).
(ii) HHAs must submit the reconsideration extension request no later than 30 calendar
days from the date of the written notification of noncompliance.
(iii) The reconsideration extension request must—
(A) Be submitted to CMS via email to CMS HHAPU reconsiderations at
HHAPUReconsiderations@cms.hhs.gov; and
(B) Contain all the following information:
(1) The CCN for the HHA.
(2) The business name of the HHA.
(3) The business address of the HHA.
(4) Contact information for the HHA’s chief executive officer or designated personnel,
including the name, telephone number, title, email address, and physical mailing address, which
may not be a post office box.
(5) A statement of the reason for the request for the extension.
(6) Evidence of the impact of extraordinary circumstances, including, for example,
photographs, newspaper articles, and other media.
(6) CMS notifies the HHA in writing of its final decision regarding the HHA’s request
for an extension to file a reconsideration of noncompliance request via an email from CMS.
* * * * *
32. Section 484.358 is amended by adding paragraph (i) to read as follows:
Update to all-payer data submission of OASIS
CMS finalized technical changes to the Conditions of Participation (CoPs).
- § 484.45(a) will state that an HHA must encode and electronically transmit each completed OASIS assessment to the CMS system, “regarding each patient” with respect to which information is required to be transmitted (as determined by the Secretary), within 30 days of completing the “assessment of the patient” and § 484.55(d)(1)(i) will state “Elected transfer.”
- The requirement for reporting OASIS information applies to all HHA patients receiving skilled services and aligns the language in the CoPs with the requirements finalized in the CY 2023 and CY 2025 HH PPS final rules.
CMS states there is no change to existing policy regarding patient exemptions from OASIS, which are as follows: patients under the age of 18; patients receiving maternity services; and patients receiving only personal care, housekeeping, or chore services.
Additionally, providers are reminded that the OASIS submission requirements continue not to apply to patients receiving Part B outpatient therapy services provided by an HHA that elects to provide these outpatient services. Patients receiving Part B outpatient therapy services would not have an HHA plan of care nor would an OASIS assessment be completed on these patients.
The updated regulatory text will appear as follows:
§ 484.55 Condition of participation: Comprehensive assessment of patients.
* * * * *
(d) * * *
(1) * * *
(i) Elected transfer;
* * * * *
31. Section 484.245 is amended by revising paragraph (d)(4) and adding paragraphs (d)(5) and (6) to read as follows:
Revised HH CAHPS Survey
CMS is finalizing update of the HH CAHPS measures beginning with the April 2026 sample month per the content below:
- No survey administration changes with the new survey
- The revised survey is shorter than the current survey
- It includes changes to the survey and the quality measures derived from testing include the following:
- Addition of three new questions to assess new topics of importance to patients
- ++ Whether the care provided helped the patient take care of their health
- ++ Whether the patient’s family/friends were given sufficient information and instructions
- Removal of questions or topics of less importance to patients (that is, six questions about medications were reduced to two questions).
- Removal of questions not currently used in public reporting composites (that is, three questions on which type of staff served the patient—nurse, physical or occupational therapist, and home care aide).
- Removal of one question which did not perform well in testing to stand alone or fit into one of the revised composite measures:
- Whether the patient got information about what care and services they would get when they first started getting home health care
- Minor text changes to selected existing questions to help clarify the question or response options, based on feedback from patients
- Addition of three new questions to assess new topics of importance to patients
- Impact of survey changes on Star Ratings:
- The revised Care of Patients measure is conceptually like the current Care of Patients measure, but the change (adding two new questions and dropping one question) is substantive and the revised measure will be treated as new
- The revised Communications Between Providers and Patients measure is similar to the current Communications Between Providers and Patients measure but the change (dropping two questions and adding one new question) is substantive and the revised measure will be treated as new
- The Summary Star Ratings will continue to be calculated using four rolling quarters and will be publicly reported for all HHAs with 40 or more completed surveys over the reporting period. Star Ratings are updated every quarter.
- Case-mix variable adjustment
- The current case-mix adjustment model includes the following variables: patient age, patient education, self-reported overall health, self-reported mental health, diagnosis of schizophrenia or dementia, whether the patient lives alone, whether the patient or a proxy answered the survey, and language in which the survey was completed
- Based on testing an analysis, CMS will drop the adjustment for diagnoses of schizophrenia or dementia with the revised survey
- CMS is not adopting a web-based survey mode at this time but stated it will continue to evaluate the possibility of a web-based mode in the future.
The revised HHCAHPS Survey instrument and crosswalks between the original and proposed publicly reported measures are available on the HHCAHPS website at https://homehealthcahps.org/Survey-and-Protocols/Survey-Materials
Policies Regarding Public Display of Measure Data for the HH QRP
CMS anticipates the first Care Compare refresh in which publicly reported measures scores would be updated to include the new measures would be October 2027, with scores calculated using data from Q2 2026 through Q1 2027. In the interim period, measure scores will be made available to HHAs confidentially via their Provider Preview reports on the HHCAHPS Survey website after two full quarters of data are submitted.
Request for Information (RFI)
Measure Concepts Under Consideration for Future Years
CMS sought public input on four concepts for future measures for the HH QRP which it intends to use to inform future measure development efforts.
- Interoperability – CMS defined “interoperability” in part, and with respect to health IT, as health IT that enables the secure exchange of electronic health information with, and use of electronic health information from, other health IT without requiring special efforts by the user.
- They requested input and comment on approaches to assessing interoperability in the HH setting, for instance, measures that address or evaluate the level of readiness for interoperable data exchange, or measures that evaluate the ability of data systems to securely share information across the spectrum of care.
- Comment summary: Comments of support were received with the qualification that federal funding was needed to ensure home health could have the same advances in health record systems seen in hospital and physician practices. Other commenters who stressed the importance of interoperability also noted that they didn’t support an interoperability measure concept for HHA due to what they described as significant financial and operational barriers to advancing standardized interoperability in HH.
- Cognitive Function – This RFI is requested input on cognitive functioning measures that may be available for immediate use, or that may be adapted or developed for use in the HH QRP, using the BIMS or the CAM measurement tools.
- CMS also sought input on the feasibility of measuring:
- improvement in cognitive functioning during a HH stay, which typically averages 56 days
- the cognitive skills (example executive functions) that are more likely to improve during an HHA stay
- conditions for which measures of maintenance – rather than improvement in cognitive functioning – are more practical
- the types of intervention that have been demonstrated to assist in improving or maintaining cognitive functioning
- Comment summary: Many commenters emphasized that CMS should prioritize measures aimed at maintaining cognitive function or limiting cognitive decline, rather than focusing solely on improvement. Given the complexity of cognitive function, several respondents recommended against developing a single measure for this domain or advised postponing such development considering current challenges.
- CMS also sought input on the feasibility of measuring:
- Well-Being – CMS sought input on a quality measure concept of nutrition specifically feedback on tools and frameworks that promote healthy eating habits, exercise, nutrition, or physical activity for optimal health, well-being, and best care for all.
- Comment summary: Many commenters suggested that given the timeframe of a home health stay, the focus for CMS should be on a process rather than outcome measure since the HHA would have limited ability to affect the broad concept of well-being. Others cautioned that HHAs could not address an issue as broad as well-being in the timeframe of a patient’s HH care and that this measure concept should not be considered for the HH QRP.
- Nutrition – CMS sought input on a quality measure concept of nutrition. Assessment of nutritional status may include various strategies, guidelines, and practices designed to promote healthy eating habits and ensure individuals receive the necessary nutrients for maintaining health, growth, and overall well-being. This also includes aspects of health that support or mediate nutritional status, such as physical activity and sleep.
- Comment summary: Commenters who supported developing a nutrition measure concept stressed the need for using tools that were validated and reliable. This group also stressed that a measure concept related to nutrition should be a process measure because of the complex range of issues that encompass nutrition issues.
- Commenters who did not support current development of a nutrition measure concept cited the lack of reimbursement for services that would support nutrition interventions. They also noted that the complex issues around nutrition care would not fall within the scope of HHAs to address in the limited time frame of home health care.
Potential Revision of the Final Data Submission Deadline Period from 4.5 Months to 45 Days
CMS sought feedback on a potential change to the final data submission deadline from 4.5 months to 45 days after the close of the period which they intend to use to inform program improvement efforts.
Comment summary: Most commenters supported a reduction in the final data submission deadline from 4.5 months, with additional recommendations related to implementation. They suggested that CMS should pilot the reduction in submission deadlines before moving to national implementation of the policy update, stating that this would allow CMS to evaluate the impacts and determine appropriate technical guidance, stakeholder engagement, and operational flexibility needed to successfully implement this change.
Other commenters cautioned that a transition from 4.5 months to 45 days would cause harm to a range of HHAs due to additional administrative burden. They noted that this would especially be the case for small HHAs, stand-alone HHAs that operate with limited resources, HHAs that manage coding and review in-house, HHAs experience high field staff turnover, or HHAs that lack robust EMR or analytics systems. They noted this could introduce errors and comprise the quality of OASIS data submitted.
CMS thanked all the commenters for responding to the proposed measure concept RFI and stated while they are not responding to specific comments in response to the RFI in this final rule, we will take this feedback into consideration for our future measure development efforts for the HH QRP.
Advancing Digital Quality Measurement in the HH QRP
As part of an effort to advance the digital quality measurement (dQM) transition, CMS issued a request for information (RFI) in the proposed rule to gather broad public input on the dQM transition in HHAs. They sought feedback on the current state of health IT use, including electronic health records (EHRs), in HHAs.
Comment summary: Many commenters expressed support for a transition to dQMs in the HH QRP, citing that using FHIR as a standard can alleviate administrative burden and improve data quality if implemented effectively. Many of these commenters supported the transition but had recommendations for CMS on successful implementation for HHAs, including a phased implementation or “glide path” approach, reporting flexibility, and adequate time to update systems after CMS finalizes a change to HH QRP requirements.
Many commenters recommended funding or incentive opportunities to obtain resources and technology for improved exchange of health information. Numerous commenters also noted that implementation and updating EHRs is resource intensive, and that HHAs, along with other PAC providers, were not included in Meaningful Use funding through the Health Information Technology for Economic and Clinical Health (HITECH) Act of 2009.
CMS thanked commenters for their feedback and stated while they will not be responding to specific comments submitted in response to this RFI in this final rule, they intend to use this
Expanded Home Health Value-Based Purchasing (HHVBP) Model
Changes to HHVBP Measure Removal Factors
CMS proposed and invited public comment about adding and codifying an additional measure removal factor at § 484.358, Factor 9: It is not feasible to implement the measure specifications.
They noted this new measure removal factor would enable them to address situations in which it is no longer feasible to continue implementing a quality measure, such as when a data collection instrument is revised in a way that no longer collects the information required for the quality measure specifications.
CMS is finalizing the addition and to codify at § 484.358 Measure Factor 9: It is not feasible to implement the measure specifications.
Changes to the Applicable Measure Set
After consideration of the public comments received, CMS finalized the removal of three HHCAHPS Survey-based measures as follows:
- Care of Patients
- Communications between Providers and Patients
- Specific Care Issues
Also, four of the seven survey items used to calculate the Specific Care Issues measure will be removed because the survey changes have been finalized, making it impossible to calculate the measure as currently specified.
Removal of data elements associated with the HH QRP would begin with assessments as of April 1, 2026.
The updated regulatory text will appear as follows:
§ 484.358 HHVBP Measure removal factors.
* * * * *
(i) It is not feasible to implement the measure specifications.
Addition of OASIS-Based Function Measures to the Expanded HHVBP Model Applicable Measure Set
- After consideration of the public comments received, CMS is finalizing the addition of the three OASIS-based function measures, and one claims based measure to the HHVBP applicable measure set.
- Three OASIS-based measures related to bathing and dressing beginning with CY 2026:
- Improvement in Bathing (based on OASIS item M1830)
- Improvement in Upper Body Dressing (based on OASIS item M1810)
- Improvement in Lower Body Dressing (based on OASIS item M1820)
- One claims-based measure, the Medicare Spending per Beneficiary for the Post-Acute Care (PAC) setting measure
- Three OASIS-based measures related to bathing and dressing beginning with CY 2026:
Updates to Individual Measure Weights and Category Weights
CMS proposed and requested public feedback about revising the weights of the individual measures starting with the CY 2026 performance year as well as revising the measure category weights.
After consideration of the public comments received, CMS is finalizing the changes to the expanded HHVBP Model’s individual measure weights and category weights as follows:
- CMS will adjust the measure category weights for the larger-volume cohort such that the OASIS-based and claims-based measure categories each contribute 40%, and the HHCAHPS Survey-based measure category contributes 20% to the TPS due to the reduction in the number of individual HHCAHPS Survey-based measures.
- Changes to the applicable measure set would increase the number of OASIS-based measures from three measures to six and increase the number of claims-based measures from two to three. The number of individual measures for the HHCAHPS Survey-based measures would decrease from five to two.
HHVBP Quality Measure Concepts Under Consideration for Future Years – Request for Information (RFI)
CMS requested feedback about the addition of a respecified Falls with Major Injury measure as well as two potential changes to the HH CAHPS survey-based measures scoring rules and applicable measure set as they relate to the expanded HHVBP Model as follows:
Falls with Major Injury Measure (OASIS-based and Claims-based)
CMS reported that a recent study found that more than half of falls with a major injury identified using Medicare claims data) were not reported on OASIS assessments. OIG observed that a low fall rate reported on Care Compare may reflect a provider’s lack of falls reporting, rather than a low incidence of falls among its patients. OIG further observed that HHAs with low falls with major injury rates on Care Compare were more likely than other HHAs not to report falls among patients enrolled in Medicare.
CMS is currently working on a respecified version of the FMI measure that uses fee-for-service claims, encounter data, and OASIS data. Using multiple data sources will produce a more robust and complete data set, allowing the respecified FMI measure to be more accurate and include more providers.
CMS requested comments related to the potential addition of the respecified FMI measure to the measure set for the expanded HHVBP Model.
Potential Future Changes to HH CAHPS Scoring Rules and Applicable Measure Set
CMS sought public comments on the possibility of initially measuring HHA performance on the future HH CAHPS Survey-based measures based solely on achievement, rather than both achievement and improvement.
Adding to the Applicable Measure Set for the Expanded HHVBP Model the Three Remaining Items in the Specific Care Issues Measure as Single Item Measures
CMS proposed to modify the HHCAHPS Survey instrument. Among other changes, this proposal would remove several items used in the multi-item Specific Care Issues measure. Three of the items used in the Specific Care Issues measure will remain in the HHCAHPS Survey instrument. The three items from the Specific Care Issues measure included in the revised HHCAHPS Survey instrument are as follows:
- When you first started getting home health care from this agency, did someone from the agency talk about ways to help make your home safer?
- Has someone from the agency ever reviewed the prescribed and over-the-counter medicines you were taking?
- In the last 2 months of care, did home health staff from this agency talk with you about any side effects of your medicines?
CMS stated appreciation for the public comments submitted in response to this RFI and they will consider this input while continuing to refine the expanded HHVBP Model in the future.
Overall Impact of the Final Rule
The following are highlights in this section of the final rule and does include all content. Please review the overall impact section in the final rule in its entirety and carefully to determine all impact.
- Economic – aggregate payments in CY 2026 would decrease by 1.3 percent which reflects the -0.9 percent decrease from the permanent adjustment, the -2.7 percent decrease from the temporary adjustment, the -0.1 percent decrease from the updated FDL and the 2.4 percent home health payment update. The combined effects of all changes vary by specific types of providers and by location.
- Some individual HHAs within the same group may experience different impacts on payments than others due to the distributional impact of the CY 2026 wage index, the percentage of total HH PPS payments that were subject to the LUPA or paid as outlier payments, and the degree of Medicare utilization. (See Table 51: CY 2026 HHA Impacts By Facility Type And Area Of The Country, page 648 for details)
- HH QRP for CY 2027 – CMS will remove four standardized patient assessment data elements in CY 2026: Living Situation, Food Runs Out, Food Doesn’t Last, and Utilities. The COVID-19 Vaccine: Percent of Patients Up to Date measure and its related item will also be removed.
- The net effect of these proposals is a decrease of four data elements at the start of care and resumption of care time points and a decrease in one data element at the transfer of care, death at home and discharge time points for a net decrease in burden.
- The implementation of provisions outlined in this final rule for the HH QRP is estimated to decrease the burden on HHAs by $1,496 per HHA annually, or $17,810,282 for all HHAs annually.
- Expanded HH VBP Model – CMS estimated the expanded HHVBP Model would generate a total projected 5-year gross FFS savings of $3,376,000,000. The changes to the applicable measure set proposed in this rule would not change those estimates because they do not change the number of HHAs in the Model or the payment methodology.
- Tables 52 and 53 (pages 656-658) in the final rule show the value-based incentive payment adjustments for the estimated 7,061 HHAs that would qualify to compete in the expanded Model based on CY 2023 performance data stratified by volume-based cohort.
- Updates to the Home Health Agency Conditions of Participation (CoPs) to Align with the OASIS All-Payer Submission Requirements – there is no projected additional burden.
See TABLE 1: SUMMARY OF ECONOMIC COSTS AND TRANSFERS, BY PROPOSED PROVISION on page pages 21-22 for more detail on impact.
Review the CMS summary of the HHA Final rule at https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2026-home-health-prospective-payment-system-final-rule-cms-1828-f