
Compliance Monitor (07/14/2025)
Your source for federal updates
July 2025 Compliance Activity
| CMS Proposed Home Health Payment Update Rule (NPRM) Expected posting in late June – early July Rule was posted – see below | Annual CY issuance May include proposed regulatory information not included in the FY hospice payment update proposed rule Subscribe to the Federal Register for emails of newly posted regulations. The selection of content can be customized. Link to subscribe is listed on the following webpage: https://www.federalregister.gov/documents/current Will first appear on the Federal Register Public Inspection Desk https://www.federalregister.gov/pu blic-inspection/current Will move over to the Federal Register within 7 days of initial posting https://www.federalregister.gov/do cuments/current |
| Transition to All-Payer OASIS Data Collection and Submission July 1, 2025 | Collect and submit OASIS data for all patients with any pay source who are not exempt from OASIS data collection and who begin receiving home health care services with an OASIS SOC M0090 date on or after July 1, 2025 https://www.cms.gov/files/document/oasisall-payer-transition-fact-sheetdec-2024.pdf |
| 1557 compliance date correction July 5, 2025 | Covered entities are required to distribute a new notice to inform individuals of the availability of non-English assistance. The new notice replaces the old foreign language “taglines” that were required under previous versions of the Section 1557 regulations. Some employers have already redesigned their notices to meet this requirement when they met earlier Section 1557 notice deadlines in the fall. |
| CMS Proposed Home Health Payment Update Rule (NPRM) Annual CY issuance May include proposed regulatory information not included in the FY hospice payment update proposed rule Subscribe to the Federal Register for emails of newly posted regulations. The selection of content can be customized. Link to subscribe is listed on the following webpage: https://www.federalregister.gov/documents/current | Usually posted in late June – early July Will first appear on the Federal Register Public Inspection Desk https://www.federalregister.gov/pu blic-inspection/current Will move over to the Federal Register within 7 days of initial posting https://www.federalregister.gov/do cuments/current |
Top Items
Notice of Proposed Rulemaking – Comment Period Ends on August 29th, 2025.
The Calendar Year (CY) 2026 Home Health PPS Notice of Proposed Rulemaking (NPRM) was displayed on June 30th, 2025. The proposed rule can be downloaded from the Federal Register at https://www.govinfo.gov/content/pkg/FR-2025-07-02/pdf/2025-12347.pdf
The content is for home health, home infusion therapy and DMEPOS providers.
Please visit the Federal Register to submit public comments. See CMS summary of proposed provisions. Comments are due by August 29; see the proposed rule for details on how to submit them.
Review the CHAP summaries of the rule:
Alert: Medicare Fraud Scheme Involving Phishing Requests Via Fax and Other Means
CMS has identified a fraud scheme targeting Medicare providers and suppliers. Scammers are impersonating CMS and sending phishing requests for medical records or payment of alleged Medicare debts, often via fax or email, falsely claiming to be part of a Medicare audit or debt collection efforts.
Important: CMS generally doesn’t initiate audits via fax or email unless a provider requests it, and Medicare overpayment collections are handled through an established process through the Medicare Administrative Contractors (MACs). Protect your information. If you receive a suspicious request, don’t respond. If you think you got a fraudulent or questionable request, work with your Medical Review Contractor to confirm if a medical records request is real or your MAC for overpayment collections.
Department of Labor Proposes Reinstating Companionship and Live-In Care Exemptions
The U.S. Department of Labor (DOL) has released a Notice of Proposed Rulemaking that would restore key exemptions under the Fair Labor Standards Act (FLSA) for home care providers. If finalized, this rule would reinstate the companionship services and live-in domestic service exemptions that were in place prior to 2013.
- The proposed changes seek to restore the ability of third-party employers (like home care agencies) to classify caregivers as exempt from the FLSA’s minimum and overtime wage requirements under the companionship and live-in domestic service exemptions.
- Home based care providers are strongly encouraged to carefully review the rule and submit comments which are due by September 2, 2025.
Review the proposed rule in the Federal Register
Hospice/Palliative Care Provider Update
Hospice Quality Reporting Program: Vendor Update Slide Deck & Errata for Data Specifications Effective October 1
CMS released a Hospice Vendor Update (PDF) slide deck and Data Submission Specifications Errata V1.00.2 (PDF) for the final Hospice Outcomes and Patient Evaluation (HOPE) data specifications V1.00.1, effective October 1, 2025. This information will help you prepare for HOPE implementation. Get more information on the HOPE Technical Information webpage.
Home Health Provider Updates
Preview Reports and Star Rating Preview Reports for the October 2025 Refresh of HH QRP Data – NOW AVAILABLE IN iQIES
The HHA Provider Preview Reports have been updated and are now available. These reports contain provider performance scores for quality measures, which will be published on the compare tool on Medicare.gov and the Provider Data Catalog (PDC) during the October 2025 refresh.
Data contained within the Provider Preview Reports are based on quality assessment data submitted by HHAs from Quarter 1, 2024 through Quarter 4, 2024. The data for the claims-based measures will display data from Quarter 1, 2023 through Quarter 4, 2024 for the Discharge to Community and Medicare Spending Per Beneficiary measures, Quarter 1, 2022 through Quarter 4, 2024 for the Potentially Preventable 30-Day Post-Discharge Readmission measure, and Quarter 1, 2024 through Quarter 4, 2024 for the Home Health Within-Stay Potentially Preventable Hospitalization measure. Additionally, the data for the HHCAHPS measures will display data from Quarter 2, 2024 through Quarter 1, 2025.
Providers have until August 8, 2025, to review their performance data. Only updates/corrections to the underlying assessment data before the final data submission deadline will be reflected in the publicly reported data on Medicare.gov. If a provider updates assessment data after the final data submission deadline, the updated data will only be reflected in the Facility-Level Quality Measure (QM) report and Patient-Level QM report. Updates submitted after the final data submission deadline will not be reflected in the Provider Preview Reports or on Medicare.gov. However, providers can request a CMS review of their data during the preview period if they believe the displayed quality measure scores within their Provider Preview Reports are inaccurate.
For questions related to accessing your facility’s Provider Preview Report, please contact the iQIES Service Center by email at iqies@cms.hhs.gov or call 1-800-339-9313. For questions about HHA Quality Reporting Program (QRP) Public Reporting, please email homehealthqualityquestions@cms.hhs.gov.
Reminder: Interim Performance Reports (IPRs) – Final April 2025 IPRs Available in iQIES
The Final April 2025 IPRs for the expanded HHVBP Model have been published in the Internet Quality Improvement and Evaluation System (iQIES).
Interim Performance Reports (IPRs) – Preliminary July 2025 IPRs Available in iQIES in July, including new information for the CY 2025 applicable measures
The Preliminary July 2025 IPRs for the expanded HHVBP Model will be published in iQIES in July. Starting with this IPR, HHAs that were initially Medicare certified in 2023 are eligible to receive IPRs. For additional details on cohort assignment in the expanded Model, please navigate to Section 2 in the Expanded HHVBP Model Guide, available on the Expanded HHVBP Model webpage, under “FAQs & Model Guide.”
An HHA receives a July 2025 IPR if the HHA:
- Was Medicare certified prior to January 1, 2024, and
- Meets the minimum threshold of data for at least one (1) quality measure in the quarterly reporting period for the performance year shown in Exhibit 1.
Exhibit 1: July 2025 IPR quality measure performance scores time periods for each measure category
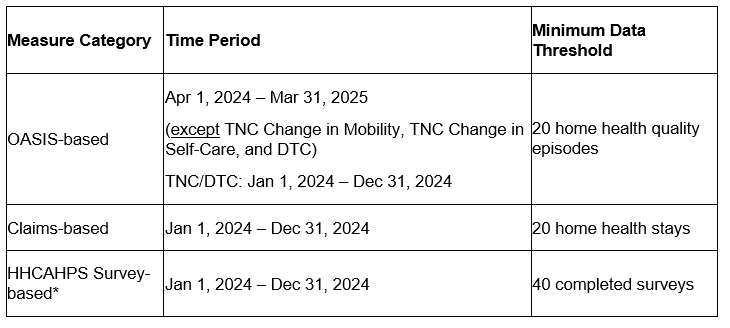
* Not included in the TPS calculation for HHAs in the smaller-volume cohort.
TNC = Total Normalized Composite. DTC = Discharged to Community.
Note: IPRs are only available to HHAs through iQIES and are located in the “HHA Provider Preview Reports” folder. IPRs are not available to the public.
As outlined in the CY 2024 Home Health (HH) Prospective Payment System (PPS) Final Rule, there are changes to the expanded HHVBP Model measure set starting with CY 2025 (referred to as the “CY 2025 measure set”). The October 2025 IPRs will be the first IPRs to generate Improvement, Achievement, and Care Points based on the CY 2025 measure set. To help HHAs get ready for this change, CMS has started providing resources, including the following three worksheets, that provide a preview of HHAs’ performance on the CY 2025 measure set:
- “CY 2025 AT and BM” tab: Starting with the July 2024 IPRs, preliminary Achievement Thresholds (AT) and Benchmarks (BM) have been available to HHAs in each IPR. In the July 2025 IPRs, this tab will show final AT and BM.
- “CY 2025 Baseline” tab: The January 2025 IPRs reported on HHAs’ preliminary Improvement Thresholds for the CY 2025 measure set. NEW – In the July 2025 IPRs, this tab will show final Improvement Thresholds.
- NEW – “CY 2025 Performance” tab: In the July 2025 IPRs, CMS Note: As some measures are included in both the CY 2024 and CY 2025 measure set, this additional tab will only report on the measures that are new to the CY 2025 measure set (i.e., Home Health Within-Stay Potentially Preventable Hospitalization, Discharge to Community-Post Acute Care Discharge Function Score).
Instructions on how to access the IPRs are also available in the Expanded HHVBP Model Reports – Access Instructions (PDF) on the Expanded HHVBP Model webpage, under “Model Reports.”
Annual Performance Reports (APR) – Preview Calendar Year (CY) 2025 APRs Available in iQIES in August
The CY 2025 APR identifies how the HHA performed on the applicable measure set based on CY 2024 performance (i.e., Performance Year 2) that is translated into a corresponding payment adjustment applied to Medicare Fee-For-Service (FFS) claims with through dates in CY 2026.
APRs are published in three (3) stages – a Preview APR, a Preliminary APR, and a Final APR.
- In August 2025, HHAs will receive their Preview CY 2025 APR. The publication of the Preview CY 2025 APR provides competing HHAs with an opportunity to review their data. During the review of the Preview APR, an HHA may submit a recalculation request within 15 calendar days after CMS issues the Preview APR if they believe there is an error.
- In October 2025, HHAs will receive their Preliminary CY 2025 APR. If an HHA disagrees with the results of CMS’s recalculation request decision that is reflected in the Preliminary APR, the HHA may submit a reconsideration request within 15 calendar days after CMS issues the Preliminary CY 2025 APR. Only HHAs that submit a recalculation request may submit a reconsideration request. An HHA may request Administrator review of a reconsideration decision within seven (7) days from CMS’ notification to the HHA contact of the outcome of the reconsideration request.
- In December 2025, CMS makes the Final CY 2025 APR available after all recalculation requests, reconsideration requests, and Administrator reviews are processed and no later than 30 calendar days before the payment adjustment takes effect in the applicable payment year (CY 2026).
For additional details on the appeals process for IPRs and APRs, please review the following resource that is available on the Expanded HHVBP Model webpage, under “Model Reports”: Expanded HHVBP Model IPR and APR Recalculation Instructions (PDF)
CMS Wants to Hear from You! Call for Information
We would like to learn more about how Home Health Agencies (HHAs) are using their Home Health Value-Based Purchasing Model (HHVBP Model) performance results in conjunction with referral sources, state Medicaid agencies, or any managed care entities.
In a May 2022 Home Health Care News article titled “What HHVBP Means for Managed Care, SNF Utilization,” the publication stated HHVBP results would translate into “improved managed care relationships and further diversion away from skilled nursing facilities (SNFs).” CMS is interested in HHAs’ success stories working with referral sources and managed care entities, and/or lessons learned that may be useful to other HHAs. These stories may be used to inform future educational resources.
Has your agency been asked to provide HHVBP performance scores during contract discussions? Has your HHA been approached by a contracted or potential managed care partner based on your HHVBP Model performance results that are publicly reported on the CMS Provider Data Catalog? Has any managed care entity or state Medicaid agency adjusted your payment rates based on your HHVBP performance results or any other quality measures? Since engagement in the expanded HHVBP Model, have you experienced fewer SNF stays after hospital (re)admissions?
This Call for Information is optional. We encourage interested HHAs to submit their stories (narratives up to three paragraphs) summarizing their experiences along with any applicable CCN(s) and contact information to the HHVBP Help Desk (HHVBPquestions@cms.hhs.gov).
Resource Spotlight – Quality Improvement Resources Available
HHAs looking for quality improvement strategies may benefit from reviewing the collection of Quality Assurance and Performance Improvement (QAPI) resources on the Expanded HHVBP Model webpage, under “Quality Improvement”.
- The QAPI resources that provide HHAs with concrete QAPI strategies and suggestions for creating action plans include the Strategies for Success Self-Assessment (Webinar | PDF) and the Briefing Card Compendium (PDF).
- Two resources feature HHA Perspectives panel series based on discussions with volunteer panelists who reviewed strategic approaches to managing and improving agency performance: Quality Management (Webinar | PDF) and Innovation (Webinar | PDF). Essentials Modules webinars focus on Patient and Family/Caregiver Engagement (Assessment and Goal Setting and Teaching and Guidance) and Care Transitions (Provider Communication and Medication Management).
- Lastly, a series of podcasts feature home health experts highlighting practices related to Leadership and Communication — Essential Elements for Quality Improvement, The Patient with Declining Memory: The “Keys” to Safe Mobility, Infection Prevention and Control: Home Health Patient Care and Communication, and Managing Chronic Illness through Home Health Care.
Note: Content included in the available QAPI resources was accurate at the time of publication and may or may not reflect more current evidence-based practices. Please use your professional and clinical judgment when incorporating new strategies or tools into your practice.
Federal Register Post – Review Choice Demonstration for Home Health Services
the CMS seeks to develop and implement a Medicare demonstration project, which CMS believes will help assist in developing improved procedures for the identification, investigation, and prosecution of Medicare fraud occurring among Home Health Agencies (HHA) providing services to Medicare beneficiaries. This revised demonstration helps assist in developing improved procedures for the identification, investigation, and prosecution of potential Medicare fraud. The demonstration helps make sure that payments for home health services are appropriate through either pre-claim or post payment review, thereby working towards the prevention and identification of potential fraud, waste, and abuse; the protection of Medicare Trust Funds from improper payments; and the reduction of Medicare appeals. CMS has implemented the demonstration in Illinois, Ohio, North Carolina, Florida, and Texas with the option to expand to other states in the Palmetto/JM jurisdiction. Under this demonstration, CMS offers choices for providers to demonstrate their compliance with CMS’ home health policies. Providers in the demonstration states may participate in either 100 percent pre-claim review or 100 percent post payment review. These providers will continue to be subject to a review method until the HHA reaches the target affirmation or claim approval rate. Once an HHA reaches the target pre-claim review affirmation or post-payment review claim approval rate, it may choose to be relieved from claim reviews, except for a spot check of their claims to ensure continued compliance. Providers who do not wish to participate in either 100 percent pre-claim or post payment reviews have the option to furnish home health services and submit the associated claim for payment without undergoing such reviews; however, they will receive a 25 percent payment reduction on all claims submitted for home health services and may be eligible for review by the Recovery Audit Contractors.
Form Number: CMS-10599 (OMB control number: 0938-1311); Frequency: Frequently, until the HHA reaches the target affirmation or claim approval threshold and then occasionally; Affected Public: Private Sector (Business or other for-profits and Not-for-profits); Number of Respondents: 4,700; Number of Responses: 3,173,016; Total Annual Hours: 1,600,608. (For questions regarding this collection contact Jennifer McMullen (410)786-7635.)
DME Provider Updates
Medicare Improperly Paid Suppliers for Intermittent Urinary Catheters
In a report, the Office of the Inspector General found that Medicare improperly paid for catheters and kits. To avoid improper payments, review the Urological Supplies provider compliance tip for more information, including:
- Billing codes
- Denial reasons and how to prevent them
- Refill and documentation requirements
All Providers Updates
DOJ-HHS False Claims Act Working Group
Healthcare fraud and abuse depletes taxpayer funds, corrodes public health and safety, and undermines the integrity of the federal healthcare system. This Administration is fully committed to supporting such work. HHS and DOJ’s Civil Division are strengthening their ongoing collaboration to advance priority enforcement areas through the DOJ-HHS False Claims Act Working Group. The DOJ-HHS False Claims Act Working Group encourages whistleblowers to identify and report violations of the federal False Claims Act involving priority enforcement areas.
For a PDF version, please visit: https://www.hhs.gov/sites/default/files/hhs-doj-false-claims-act-working-group.pdf [PDF, 135 KB]
CMS Launches New Model to Target Wasteful, Inappropriate Services in Original Medicare
Model will leverage enhanced technologies to protect Medicare beneficiaries, federal taxpayers from unnecessary services, fraud, waste, and abuse
The Centers for Medicare & Medicaid Services (CMS) is announcing a new Innovation Center model aimed at helping ensure people with Original Medicare receive safe, effective, and necessary care. Through the Wasteful and Inappropriate Service Reduction (WISeR) Model, CMS will partner with companies specializing in enhanced technologies to test ways to provide an improved and expedited prior authorization process relative to Original Medicare’s existing processes, helping patients and providers avoid unnecessary or inappropriate care and safeguarding federal taxpayer dollars. This model builds on other changes being made to prior authorization as announced by the U.S. Department of Health and Human Services and CMS on Monday.
To view the Model Overview fact sheet, visit: https://www.cms.gov/files/document/wiser-fact-sheet.pdf.
For more information on the WISeR Model, visit: https://www.cms.gov/priorities/innovation/innovation-models/wiser.
The WISeR Model can be seen on the Federal Register at: https://www.federalregister.gov/d/2025-12195.
The CMS Innovation Center is hosting an office hour to provide an overview of the
WISeR Model and answer questions about the model. Please see below for the office
hour registration link and model overview information.
WISeR Model Office Hour Date and Time:
Thursday, July 17, 2025, 1:00 – 2:00 PM EST
Registration is open
CMS – Wasteful and Inappropriate Service Reduction (WISeR) Model Office Hour
The WISeR Model will help protect American taxpayers by leveraging enhanced technologies, such as Artificial Intelligence (AI) and Machine Learning (ML), along with human clinical review, to ensure timely and appropriate Medicare payment for select items and services. WISeR is a voluntary model, which will run from January 1, 2026, to December 31, 2031, that promotes reducing fraud, waste, and abuse by navigating patients towards safe and evidence-supported best practices.
The Office Hour will address frequently asked questions about the model and respond to live inquiries from the audience. We encourage you to submit questions in advance using the registration form linked above.
Please share this event with colleagues who may be interested in learning more about the WISeR Model. Following the event, presentation materials will be available on the WISeR Model Webpage.
National Health Care Fraud Takedown Results in 324 Defendants Charged in Connection with Over $14.6 Billion in Alleged Fraud
The Justice Department announced the results of its 2025 National Health Care Fraud Takedown, which resulted in criminal charges against 324 defendants, including 96 doctors, nurse practitioners, pharmacists, and other licensed medical professionals, in 50 federal districts and 12 State Attorneys General’s Offices across the U.S., for their alleged participation in various health care fraud schemes involving over $14.6 billion in intended loss. The Takedown involved federal and state law enforcement agencies across the country and represents an unprecedented effort to combat health care fraud schemes that exploit patients and taxpayers.
Read the full press release.
CMS Notifies Individuals Potentially Impacted by Data Incident
CMS is notifying Medicare beneficiaries whose personal information may have been involved in a data incident affecting Medicare.gov accounts. CMS identified suspicious activity related to unauthorized creation of certain beneficiary online accounts using personal information obtained from unknown external sources. CMS takes this situation very seriously. The safeguarding and security of personally identifiable information is of the utmost importance to CMS.
Read the full press release.
Providers Accepting CHAMPVA: You Must Get Paid by EFT
If you treat patients who are covered by the Civilian Health and Medical Program of the Department of Veterans Affairs (CHAMPVA), you must sign up for direct deposit (electronic funds transfer (EFT)) to get paid. Getting paid by EFT isn’t optional, it’s a federal requirement. VA paused claims payments for providers who aren’t enrolled in EFT and will resume payments once they enroll.
Enrolling in EFT helps:
- Keep CHAMPVA claim payments secure, efficient, and compliant
- Protect Veterans’ family members’ access to benefits
2 steps to enroll in EFT:
- Visit the VA Financial Services Center Customer Engagement Portal
- Complete the Payment Account Setup webform; call Financial Services Center customer support at 877-353-9791 for help
About CHAMPVA
CHAMPVA is a health care program for qualified spouses, widows(ers), and children of eligible Veterans. Through CHAMPVA, VA shares the cost of certain health care services and supplies with eligible beneficiaries.
For more information, visit the CHAMPVA–Information for Providers webpage.
OSHA Registration is open! Join up for Safe + Sound Week.
You can register today to take part in Safe + Sound Week, August 11-17. Join businesses across the country who are committed to keeping workers safe through effective safety and health programs. This year’s theme is Preparedness is Your Superpower. Find materials about emergency action plans, activities for preparedness at work and at home, and more on the Plan & Promote Your Participation webpage
Educational Opportunity
Next CMS Home Health, Hospice & Durable Medical Equipment Open Door Forum
Wednesday, August 7, 2024- Agenda: Home Health, Hospice & DME Open Door Forum (PDF)