
CMS Posts FY 2026 Hospice Wage Index Proposed Rule
The Fiscal Year 2026 Hospice Wage Index and Payment Rate Update and Hospice Quality Reporting Program Requirements [CMS-1835-P] was posted on the Federal Register Public Inspection desk on 4/11/2025. The rule should be posted in the Federal Register on 4/18/2025. CMS will accept comments related to the proposed rule through June 10, 2025, 11:59 pm. Information about submitting comments appears at the beginning of the rule.
Providers are strongly encouraged to review the entire content of the proposed rule as not all detailed information is included in this summary. Here are the highlights of the proposed rule.
Payment update information:
- The FY 2026 proposed hospice payment update percentage is 2.4%.
- The proposed 2.4 percent hospice payment update percentage for FY 2026 is based on a proposed 3.2 percent inpatient hospital market basket percentage increase for FY 2026, reduced by a proposed 0.8 percentage point productivity adjustment.
- Estimated net impact of the policies in this rule is approximately $695 million in increased revenue to hospices in FY 2026.
- Hospices in urban areas would experience, on average, a 2.4 percent increase in estimated payments compared to FY 2025; while hospices in rural areas would experience, on average, a 2.7 percent increase in estimated payments compared to FY 2025.
- Hospices providing services in the New England region would experience the largest estimated increase in payments of 3.8 percent.
- Hospices serving patients in the Pacific region will experience, on average, the lowest estimated increase of 1.4 percent in FY 2026 payments.
- This proposed rule includes the proposed updates to the hospice wage index and makes the application of the updated wage data budget neutral for all four levels of hospice care.
- CMS also proposes that if more recent data becomes available after the publication of this proposed rule and before the publication of the final rule (for example, a more recent estimate of the inpatient hospital market basket percentage increase or productivity adjustment), CMS would use such data, if appropriate, to determine the hospice payment update percentage in the FY 2026 final rule.
- They continue to believe it is appropriate to routinely update the hospice payment system so that it reflects the best available data regarding differences in patient resource use and costs among hospices as required by the statute.
- The proposed hospice wage index applicable for FY 2026 (October 1, 2025 through September 30, 2026) is available on the CMS website for the FY 2026 Hospice Wage Index proposed rule at https://www.cms.gov/medicare/payment/fee-for-serviceproviders/hospice/hospice-regulations-and-notices
- For FY 2026, CMS proposes to rebase and revise the IPPS market basket to reflect a 2023 base year.
- The proposed hospice cap amount for the FY 2026 cap year is $35,292.51.
- CMS also proposes that if more recent data becomes available after the publication of this proposed rule and before the publication of the final rule (for example, a more recent estimate of the hospice payment update percentage), we would use such data, if appropriate, to determine the hospice cap amount in the FY 2026 final rule.
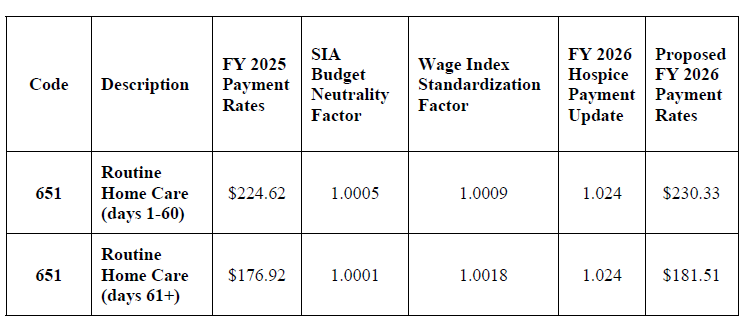
Proposed FY 2026 Hospice RHC Payment Rates
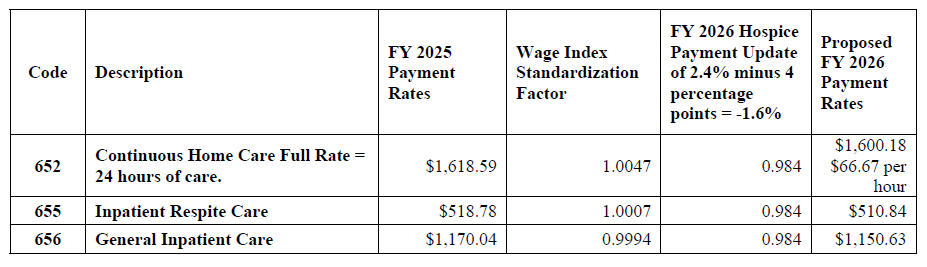
Proposed FY 2026 Hospice CHC, IRC, and GIP Payment Rates

Proposed FY 2026 Hospice RHC Payment Rates for Hospices That DO NOT Submit the Required Quality Data (the proposed FY 2026 hospice payment update percentage of 2.4 percent minus 4 percentage points)
| Code | Description | FY 2025 Payment Rates | SIA Budget Neutrality Factor | Wage Index Standardization Factor | FY 2026 Hospice Payment Update of 2.4% minus 4 percentage points = – 1.6% | Proposed FY 2026 Payment Rates |
| 651 | Routine Home Care (days 1-60) | $224.62 | 1.0005 | 1.0009 | 0.984 | $221.34 |
| 651 | Routine Home Care (days 61+) | $176.92 | 1.0001 | 1.0018 | 0.984 | $174.42 |
Proposed FY 2026 Hospice CHC, IRC, and GIP Payment Rates for Hospices That DO NOT Submit the Required Quality Data (the proposed FY 2026 hospice payment update percentage of 2.4 percent minus 4 percentage points)
Proposed Regulation Change to Admission to Hospice Care
Background –
In the FY 2025 Hospice Wage Index and Rate Update final rule (89 FR 64231), CMS received several comments requesting that the physician member of the IDG be added to the hospice admission regulation at § 418.25. Specifically, commenters requested that the language regarding which physicians can make determinations for hospice admission align with current certification requirements and CoPs. CMS did not make a change to § 418.25 in the FY 2025 hospice final rule as they did not propose this change.
The change –
- To align with the current payment and CoP regulations at §§ 418.22(c)(1)(i) and 418.102(b), respectively, CMS proposes to add the text “or the physician member of the hospice interdisciplinary group” at § 418.25(a) and (b) to indicate that, in addition to the medical director or physician designee, the physician member of the hospice IDG may also determine admission to hospice care.
- They believe aligning the language at § 418.25(a) and (b) with the language at §§ 418.102(b) and 418.22(c)(1)(i) would allow for greater consistency between key components of hospice regulations and policies.
Proposed updated regulatory text
§ 418.25 Admission to hospice care.
(a) The hospice admits a patient only on the recommendation of the medical director (or
the physician designee, as defined in § 418.3) or the physician member of the hospice
interdisciplinary group, in consultation with, or with input from, the patient’s attending physician (if any).
(b) In reaching a decision to certify that the patient is terminally ill, the hospice medical
director (or the physician designee, as defined in § 418.3) or the physician member of the
hospice interdisciplinary group, must consider at least the following information:
* * * * *
Proposed Clarifying Regulation Change Regarding Face-to-Face Attestation
Background –
- As explained in the CY 2011 HH PPS final rule, the regulation at § 418.22(b)(4) set forth that the physician or NP who performs the face-to-face encounter with the patient must attest in writing that he or she had a face-to-face encounter with the patient and, at that time, set forth that the attestation of the nurse practitioner shall state that the clinical findings of that visit were provided to the certifying physician, for use in determining whether the patient continues to have a life expectancy of 6 months or less, should the illness run its normal course. Further, the regulation set forth that the attestation, its accompanying signature, and the date signed must be a separate and distinct section of, or an addendum to, the recertification form, and must be clearly titled (75 FR 70463).
- In the FY 2012 Hospice Wage Index final rule (76 FR 47314), CMS finalized that any hospice physician can perform the face-to-face encounter regardless of whether that physician recertifies the patient’s terminal illness and composes the recertification narrative. Additionally, we amended the regulatory text at § 418.22(b)(4) to provide that the attestation of the NP or a non-certifying hospice physician shall state that the clinical findings of that encounter were provided to the certifying physician, for use in determining continued eligibility for hospice. However, they unintentionally omitted from the regulatory text at § 418.22(b)(4) the explicit requirements that the attestation include the accompanying signature of the practitioner who performed the face-to-face encounter, and the date signed.
The change –
- CMS proposes to amend § 418.22(b)(4) to set forth that the physician, or NP who performs the face-to-face encounter attest that the face-to-face encounter occurred, and the attestation must include the signature of the physician or NP who conducted the face-to-face encounter and the date it was signed.
- Further, CMS proposes that the attestation, its accompanying signature, and the date signed, must be a separate and distinct section of, or an addendum to, the recertification form, and must be clearly titled.
Proposed updated regulatory text
§ 418.22 Certification of terminal illness.
* * * * *
(b) * * *
(4) The physician or nurse practitioner who performs the face-to-face encounter with the patient described in paragraph (a)(4) of this section must attest in writing that he or she had a face-to-face encounter with the patient, including the date of that visit. The attestation must include the physician’s or nurse practitioner’s signature and the date it was signed. The attestation, its accompanying signature, and the date signed, must be a separate and distinct section of, or an addendum to, the recertification form, and must be clearly titled. If the face-to-face encounter was not performed by the certifying physician, the attestation of the physician or nurse practitioner who performed the face-to-face encounter shall state that the clinical findings of that visit were provided to the certifying physician for use in determining continued eligibility for hospice care.
* * * * *
Proposed Revision to § 418.312(j)(2) to Correct Regulatory Text
CMS proposes to revise the regulatory text at § 418.312(j)(2) to correct a reference to another part of the regulations. Specifically, CMS is replacing a reference to § 412.306(b)(2) with the correct reference to § 418.306(b)(2).
§418.312 Data submission requirements under the hospice quality reporting program.
* * * * *
(j) * * *
(2) A hospice must meet or exceed the data submission compliance threshold in
paragraph (j)(1) of this section to avoid receiving a 4-percentage point reduction to its annual
payment update for a given FY as described under § 418.306(b)(2).
Updates to the Hospice Quality Reporting Program
HOPE assessment tool
- In the FY 2025 Hospice Wage Index and Payment Rate Update final rule (86 FR 64202), CMS finalized the HOPE tool to replace the HIS as part of the HQRP.
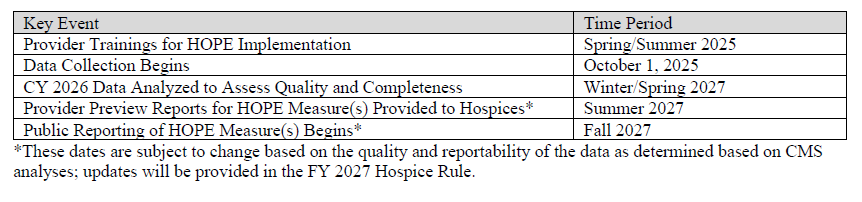
- As finalized in the FY 2025 Hospice Wage Index final rule (89 FR 64202), public reporting of the HOPE quality measures will be implemented no earlier than FY 2028. Data collected by hospices during the four quarters of CY 2026 (for example, Q 1, 2, 3 and 4 CY 2026) will be analyzed starting in CY 2027.
- CMS will inform the public of the decisions about whether CMS will report some or all of the quality measures publicly based on the findings of analysis of the CY 2026 data through future rulemaking.
- Providers will have the opportunity to preview HOPE data before it is publicly reported, with the first HOPE-based QM public reporting anticipated to be no earlier than November 2027 (FY 2028).
Anticipated HOPE Public Education, Data Collection, and Reporting
Update on the Transition to iQIES
Background –
Hospices are currently required to submit HIS data to CMS using the Quality Improvement and Evaluation System (QIES) Assessment and the Submission Processing (ASAP) system and obtain reports in the Certification and Survey Provider Enhanced Reports (CASPER) system. The FY 2020 Hospice Wage Index and Payment Rate Update final rule (84 FR 38484) finalized the proposal to migrate to a new single CMS submission and reporting system.
- Beginning on October 1, 2025, the new CMS submission and reporting system will begin accepting the data from HOPE, in line with the start of HOPE data collection.
- Provider reports will also be available in this system beginning October 1, 2025.
- The QIES system will stop accepting HIS records for hospice admissions and discharges that occurred prior to October 1, 2025, including any corrections, on February 15, 2026.
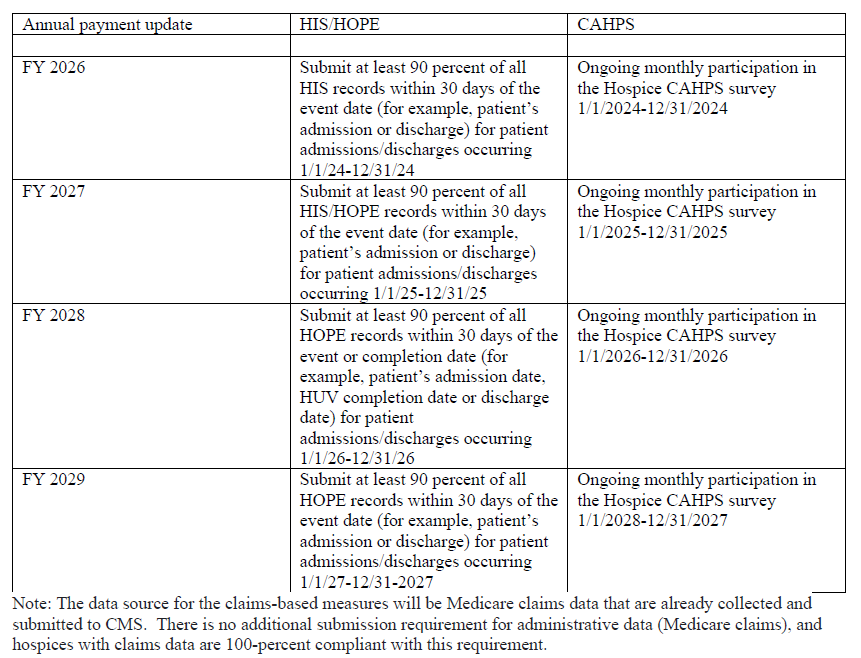
HQRP Compliance Checklist
Requests for Information (RFIs)
- Request for Information (RFI) to Advance Digital Quality Measurement (dQM) in the HQRP
Background –
- CMS is committed to improving healthcare quality through measurement, transparency, and public reporting of quality data, and to enhancing healthcare data exchange by promoting the adoption of interoperable health information technology (IT) through Health Level Seven® (HL7®) Fast Healthcare Interoperability Resources® (FHIR®) standards.
- Proposing requirements around the use of technology utilizing such standards within the HQRP in the future could potentially enable greater care coordination and information sharing, which is essential for delivering high-quality, efficient care and better outcomes at a lower cost.
- CMS is continuing to consider opportunities to advance FHIR-based reporting of patient assessment data in settings that were not eligible to participate in the Medicare Electronic Health Record (EHR) Incentive Program (now known as the Medicare Promoting Interoperability Program), while acknowledging that such providers may be at different levels of health IT adoption and readiness.
- Specifically, they are interested in assessing the feasibility of using the FHIR standard for the submission of HOPE data.
- The objective is to explore how hospices typically integrate technologies with varying complexity into existing systems and how this affects hospice workflows.
- CMS seeks to identify the challenges or opportunities that may arise during this integration, and determine the support needed to complete and submit HOPE in ways that protect and enhance care delivery.
- CMS seeks comments on a variety of questions regarding the current state of health IT use, including EHRs, in hospice facilities/organizations.
- See specific RFI questions in the proposed rule under 7b, page 41.
- RFIs on Future Quality Measure Concepts for the Hospice QRP
CMS seeks input on the importance, relevance, appropriateness, and applicability of the following concepts under consideration for future years in the Hospice QRP.
- Interoperability
- CMS seeks comments on a measure of interoperability, focusing on systems readiness and capabilities in the Hospice setting.
- The definition of interoperability cited in the proposed rule in part, and with respect to health information technology, as health information technology that enables the secure exchange of electronic health information with, and use of electronic health information from, other health information technology without requiring special efforts by the user. Adoption and optimization of electronic health records
- (EHRs) and health information exchange services that use common standards to share data can enable interoperability across systems.
- They would like to receive input and comment on approaches to assessing interoperability in the hospice care setting, for instance, measures that address or evaluate the level of readiness for interoperable data exchange, or measures that evaluate the ability of data systems to securely share information across the spectrum of care.
- Well-being
- CMS seeks feedback on a measure of well-being.
- Well-being is a comprehensive approach to disease prevention and health promotion, as it integrates mental, social, and physical health while emphasizing preventative care to proactively address potential health issues. This comprehensive approach emphasizes person-centered care by promoting the well-being of hospice patients.
- They seek comments on tools and measures that assess overall health, happiness, and satisfaction at the end of life, which could include aspects of emotional well-being, social connections, purpose, fulfillment, and self-care work.
- Nutrition
- CMS seeks feedback on a measure of nutrition.
- Assessment of nutritional status may include various strategies, guidelines, and practices designed to promote nutrition at every stage of hospice care and ensure patients receive the necessary nutrients for maintaining their individual health needs and overall well-being.
- This also includes aspects of health that support or mediate nutritional status, such as activity and sleep.
- These efforts not only support nutrition but could also work to include the cultural, social, and spiritual needs and wishes of the patients.
- CMS wants feedback on tools and frameworks that promote healthy, safe eating habits, exercise, nutrition, and activity appropriate for optimal end-of-life care.
- Deregulation Request for Information (RFI)
On January 31, 2025, President Trump issued Executive Order (EO) 14192 “Unleashing Prosperity Through Deregulation,” which states the Administration policy to significantly reduce the private expenditures required to comply with Federal regulations to secure America’s economic prosperity and national security and the highest possible quality of life for each citizen. CMS would like public input on approaches and opportunities to streamline regulations and reduce administrative burdens on providers, suppliers, beneficiaries, and other interested parties participating in the Medicare program. CMS has made available an RFI at https://www.cms.gov/medicare-regulatory-relief-rfi
Please submit all comments in response to this RFI through the provided weblink.
View the Proposed Rule on the Federal Register’s Public Inspection Desk.
For further information, see the CMS summary of the hospice proposed rule.
Questions about the content of this rule? Contact CHAP