
CMS Posts FY 2026 Hospice Wage Index Final Rule
The Fiscal Year 2026 Hospice Wage Index and Payment Rate Update and Hospice Quality Reporting Program Requirements [CMS-1835-F] was posted on the Federal Register Public Inspection desk on 8/1/2025. The rule should be posted in the Federal Register on 8/8/2025. These regulations are effective on October 1, 2025.
Providers are strongly encouraged to review the entire content of the final rule as not all detailed information is included in this summary. Here are the highlights of the final rule.
Payment update information – Final decisions:
- Final Decisions:
- The FY 2026 final hospice payment update percentage is 2.6%.
- Estimated increase of $750 million in payments from FY 2025.
- This results from the 3.3% inpatient hospital market basket percentage increase reduced by a final 0.7 percentage point productivity adjustment, required by law.
- Hospices in urban areas would experience, on average, a 2.6 percent increase in estimated payments compared to FY 2025; while hospices in rural areas would experience, on average, a 3.0 percent increase in estimated payments compared to FY 2025.
- Hospices providing services in the New England region would experience the largest estimated increase in payments of 3.9 percent.
- Hospices serving patients in the Pacific region will experience, on average, the lowest estimated increase of 1.5 percent in FY 2026 payments.
- The hospice cap amount for FY 2026 is $35,361.44 (FY 2025 cap amount of $34,465.34 increased by the FY 2026 hospice payment update percentage of 2.6%).
Final FY 2026 Payment Rates - RHC
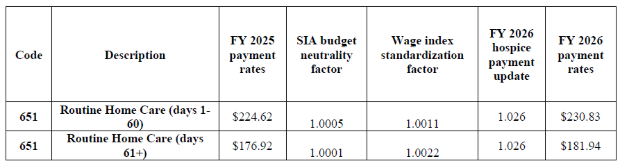
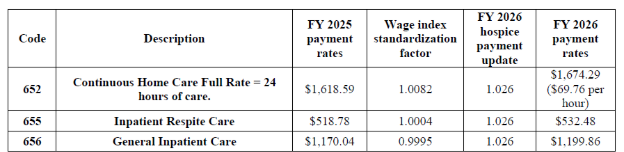
Final FY 2026 Hospice CHC, IRC, and GIP Payment Rates
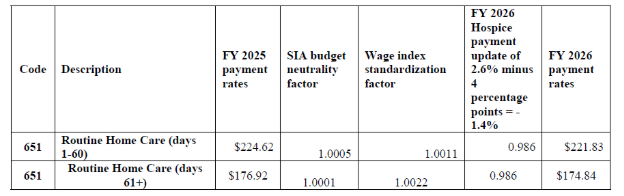
Final FY 2026 Hospice RHC Payment Rates for Hospices That DO NOT Submit the Required Quality Data (the final FY 2026 hospice payment update percentage of 2.4 percent minus 4 percentage points)
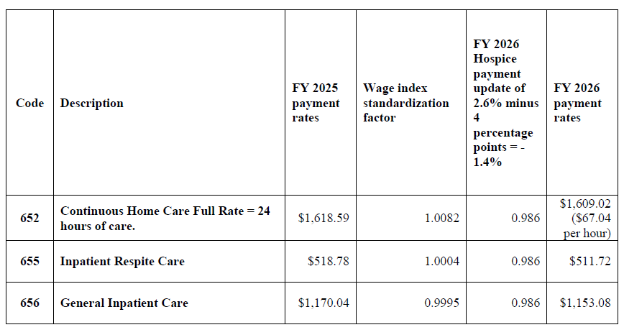
Final FY 2026 Hospice CHC, IRC, and GIP Payment Rates for Hospices That DO NOT Submit the Required Quality Data (the final FY 2026 hospice payment update percentage of 2.4 percent minus 4 percentage points)
Beginning in FY 2024 and for each subsequent year, the HHS Secretary will reduce the market basket update by 4 percentage points for any hospice that does not comply with the quality measure data submission requirements for that FY.
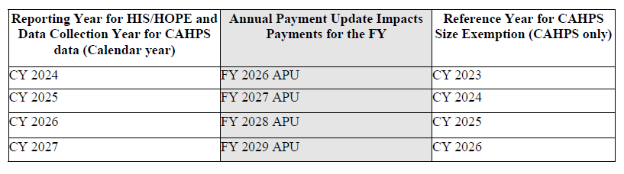
HQRP Reporting Requirements and Corresponding Annual Payments Updates.
Regulatory Text Change to Admission to Hospice Care
Background
In the FY 2025 Hospice Wage Index and Rate Update final rule (89 FR 64231), CMS received several comments requesting that the physician member of the IDG be added to the hospice admission regulation at § 418.25. Specifically, commenters requested that the language regarding which physicians can make determinations for hospice admission align with current certification requirements and CoPs. CMS did not make a change to § 418.25 in the FY 2025 hospice final rule as they did not propose this change.
Final decision
CMS is finalizing the proposal to add the text “or the physician member of the hospice interdisciplinary group” to § 418.25(a) and (b) to indicate that, in addition to the medical director or physician designee, the physician member of the hospice IDG may also determine admission to hospice care.
Final updated regulatory text
§ 418.25 Admission to hospice care.
(a) The hospice admits a patient only on the recommendation of the medical director (or
the physician designee, as defined in § 418.3) or the physician member of the hospice
interdisciplinary group, in consultation with, or with input from, the patient’s attending physician (if any).
(b) In reaching a decision to certify that the patient is terminally ill, the hospice medical
director (or the physician designee, as defined in § 418.3) or the physician member of the
hospice interdisciplinary group, must consider at least the following information:
* * * * *
Clarifying Regulation Change Regarding Face-to-Face Attestation
Background
- As explained in the CY 2011 HH PPS final rule, the regulation at § 418.22(b)(4) set forth that the physician or NP who performs the face-to-face encounter with the patient must attest in writing that he or she had a face-to-face encounter with the patient and, at that time, set forth that the attestation of the nurse practitioner shall state that the clinical findings of that visit were provided to the certifying physician, for use in determining whether the patient continues to have a life expectancy of 6 months or less, should the illness run its normal course. Further, the regulation set forth that the attestation, its accompanying signature, and the date signed must be a separate and distinct section of, or an addendum to, the recertification form, and must be clearly titled (75 FR 70463).
- In the FY 2012 Hospice Wage Index final rule (76 FR 47314), CMS finalized that any hospice physician can perform the face-to-face encounter regardless of whether that physician recertifies the patient’s terminal illness and composes the recertification narrative. Additionally, we amended the regulatory text at § 418.22(b)(4) to provide that the attestation of the NP or a non-certifying hospice physician shall state that the clinical findings of that encounter were provided to the certifying physician, for use in determining continued eligibility for hospice. However, they unintentionally omitted from the regulatory text at § 418.22(b)(4) the explicit requirements that the attestation include the accompanying signature of the practitioner who performed the face-to-face encounter, and the date signed.
Final decision
Several interested parties requested that CMS consider allowing a signed clinical note to serve as a substitute to a separate attestation that the face-to-face encounter occurred, highlighting that the statutory intent under section 1814(a)(7)(D)(i) of the Act is met if signed and dated clinical documentation clearly demonstrates that the encounter occurred and is filed in the medical record.
CMS is finalizing a modification to the regulation text at § 418.22(b)(4) to clarify that the attestation requirement may be fulfilled by not only a clearly titled section of or an addendum to the recertification form, but also by a signed and dated clinical note within the medical record that documents clear indication that the face-to-face encounter occurred and includes the date of the visit, the signature of the practitioner who conducted the face-to-face encounter, and the date of the signature.
Final updated regulatory text
§ 418.22 Certification of terminal illness.
(a) * * *
(4) * * *
(ii) During a Public Health Emergency, as defined in § 400.200 of this chapter, or through September 30, 2025, whichever is later, if the face-to-face encounter conducted by a hospice physician or hospice nurse practitioner is for the sole purpose of hospice recertification, such encounter may occur via a telecommunications technology and is considered an administrative expense. Telecommunications technology means the use of interactive multimedia communications equipment that includes, at a minimum, the use of audio and video equipment permitting two-way, real-time interactive communication between the patient and the distant site hospice physician or hospice nurse practitioner.
(b) * * *
(4) The physician or nurse practitioner who performs the face-to-face encounter with the patient described in paragraph (a)(4) of this section must attest in writing that he or she had a face-to-face encounter with the patient, including the date of that visit. The attestation must include the physician’s or nurse practitioner’s signature and the date it was signed. The attestation could be a separate and distinct section of, or an addendum to, the recertification or a clinical note that indicates the face-to-face encounter occurred, and includes the clinical findings of the face-to-face encounter, the date of the visit, the signature of the physician or nurse practitioner who conducted the face-to-face encounter, and the date of the signature. If the attestation of the nurse practitioner or a non-certifying hospice physician is a separate and distinct section of, or an addendum to, the recertification, the attestation shall state that the clinical findings of that visit were provided to the certifying physician for use in determining continued eligibility for hospice care.
Final Revision to § 418.312(j)(2) to Correct Regulatory Text
CMS finalized revising the regulatory text at § 418.312(j)(2) to correct a reference to another part of the regulations. Specifically, CMS is replacing a reference to § 412.306(b)(2) with the correct reference to § 418.306(b)(2).
§418.312 Data submission requirements under the hospice quality reporting program.
* * * * *
(j) * * *
(2) A hospice must meet or exceed the data submission compliance threshold in paragraph (j)(1) of this section to avoid receiving a 4-percentage point reduction to its annual payment update for a given FY as described under § 418.306(b)(2).
Updates to the Hospice Quality Reporting Program
HOPE assessment tool – No delay finalized
- In the FY 2025 Hospice Wage Index and Payment Rate Update final rule (86 FR 64202), CMS finalized the HOPE tool to replace the HIS as part of the HQRP beginning October 1, 2025.
- They will closely monitor the first quarter of HOPE data and expect providers to submit accurate and complete HOPE data beginning on October 1, 2025.
- As finalized in the FY 2025 Hospice Wage Index final rule (89 FR 64202), public reporting of the HOPE quality measures will be implemented no earlier than FY 2028.
- Data collected by hospices during the four quarters of CY 2026 (for example, Q 1, 2, 3 and 4 CY 2026) will be analyzed starting in CY 2027.
- CMS will inform the public of the decisions about whether CMS will report some or all of the quality measures publicly based on the findings of analysis of the CY 2026 data through future rulemaking.
- CMS states they will monitor the first quarter of HOPE data collection (quarter 4 of 2025) and provide sub-regulatory guidance on when public reporting of the two HOPE measures will begin.
- Providers will have the opportunity to preview HOPE data before it is publicly reported, with the first HOPE-based QM public reporting anticipated to be no earlier than November 2027 (FY 2028).
Anticipated HOPE Public Education, Data Collection, and Reporting
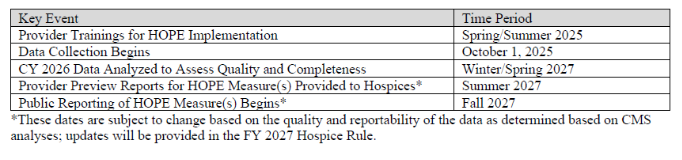
CMS posted HOPE education on the HQRP webpage as follows:
- July 15, 20205 – Hospice Outcomes and Patient Evaluation (HOPE) National Implementation Virtual Training Program Course 1: Didactic Recorded Training Series
- The slides and transcripts for these presentations can be found in the Downloads section at the bottom of the page.
- August 5, 2025 – Hospice Outcomes and Patient Evaluation (HOPE) National Implementation Virtual Training Program Course 2: Coding Workshop
Future quality measures
- As stated in the FY 2022 Hospice Wage Index final rule (86 FR 42528), CMS continues to consider developing hybrid quality measures that could be calculated from multiple data sources, such as claims, HOPE data, or other data sources (for example, CAHPS Hospice Survey).
- CMS also intends to develop several quality measures based on information collected by HOPE after HOPE is implemented. More information on measure development can be found on the HQRP Quality Measure Development web page at https://www.cms.gov/medicare/hospicequality-reporting-program/quality-measure-development.
Update on the Transition to iQIES
Background
Hospices are currently required to submit HIS data to CMS using the Quality Improvement and Evaluation System (QIES) Assessment and the Submission Processing (ASAP) system and obtain reports in the Certification and Survey Provider Enhanced Reports (CASPER) system. The FY 2020 Hospice Wage Index and Payment Rate Update final rule (84 FR 38484) finalized the proposal to migrate to a new single CMS submission and reporting system.
- Beginning on October 1, 2025, the new CMS submission and reporting system will begin accepting the data from HOPE, in line with the start of HOPE data collection.
- Providers must have access to iQIES by October 1, 2025, to submit HOPE assessments.
- CMS will provide a similar set of provider, APU, and QM reports to enable providers to monitor their data submissions.
- The iQUIES system is web-based and accessible with a single login for submitting HOPE data and accessing HOPE-related reports, replacing the current two-step process that requires two different login credentials. In addition, the new system enables a provider to have unlimited users able to submit HOPE data and access reports, with user access managed internally by a provider’s designated security official, which is designed to promote timely data submission and support APU compliance.
- The QIES system will stop accepting HIS records for hospice admissions and discharges that occurred prior to October 1, 2025, including any corrections, on February 15, 2026.
- Providers and vendors should visit the HOPE Technical Information webpage at https://www.cms.gov/medicare/quality/hospice-qualityreporting-program/hospice-outcomes-and-patient-evaluation-hope-technical-information for the latest updates and resources related to HOPE data submission specifications, including the final Hospice Outcomes and Patient Evaluation (HOPE) data submission specifications (V1.00.1) and other technical information.
- Additional questions about the transition to iQIES can be addressed to the iQIES Help Desk at iqies@cms.hhs.gov
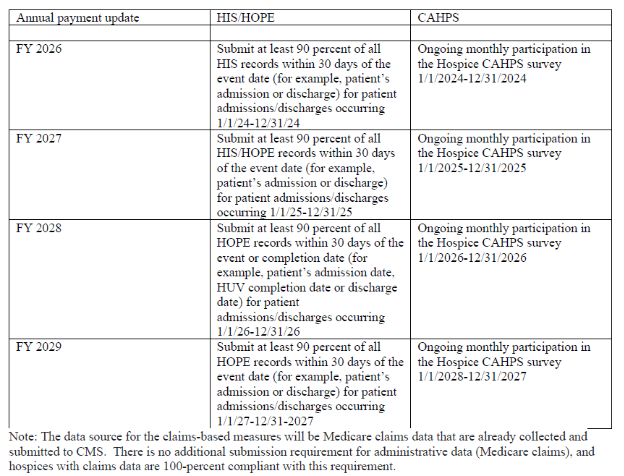
HIS/HOPE Compliance
- CMS requires that hospices submit 90 percent of all required HIS records within 30 days of the event (that is, patient’s admission or discharge).
- After HIS is phased out, hospices will continue to be required to submit 90 percent of all required HOPE records to support the quality measures within 30 days of the event or completion date (patient’s admission, discharge, and based on the patient’s length of stay up to two HUV timepoints).
- CMS states that most hospices that fail to meet HQRP requirements do so because they miss the 90 percent threshold.
- CMS offers many trainings and educational opportunities through their websites.
- https://www.cms.gov/medicare/quality/hospice
Requests for Information (RFIs)
- Request for Information (RFI) to Advance Digital Quality Measurement (dQM) in the HQRP
CMS sought comments on a variety of questions regarding the current state of health IT use, including EHRs, in hospice facilities/organizations in the proposed rule.
No comments or final decisions were provided in the final rule.
- RFIs on Future Quality Measure Concepts for the Hospice QRP
CMS asked for input on the importance, relevance, appropriateness, and applicability of the following concepts under consideration for future years in the Hospice QRP in the proposed rule on the following:
- Interoperability
- Well-being
- Nutrition
No comments or final decisions were provided in the final rule.
- Deregulation Request for Information (RFI)
CMS asked for public input related to Executive Order (EO) 14192 “Unleashing Prosperity Through Deregulation” on approaches and opportunities to streamline regulations and reduce administrative burdens on providers, suppliers, beneficiaries, and other interested parties participating in the Medicare program.
While no feedback on this request for information was provided in the final rule, CMS included the following:
Executive Order 14192, entitled “Unleashing Prosperity Through Deregulation” was issued on January 31, 2025, and requires that “any new incremental costs associated with new regulations shall, to the extent permitted by law, be offset by the elimination of existing costs associated with at least 10 prior regulations.” Therefore, this final rule is not an E.O. 14192 regulatory action since it does not impose any more than de minimis regulatory costs.
View the Final Rule on the Federal Register’s Public Inspection Desk.
For further information, see the CMS summary of the hospice final rule.
Questions about the content of this rule? Contact CHAP