
CMS Posts FY 2025 Hospice Wage Index Final Rule
The final FY 2025 Hospice Wage Index and Payment Rate Update/Quality Reporting Rule (CMS-1810-F) was posted on the Federal Register Public Inspection desk on 7/30/2024. The rule should be posted in the Federal Register on 8/6/2024. Regulations in this rule are effective on October 1, 2024, unless otherwise specified.
Providers are strongly encouraged to review the final rule as not all detailed information is included in this summary and for regulatory due dates.
Here are the highlights of the final rule
Payment update information:
- The FY 2025 final hospice payment update percentage is 2.9%.
- CMS is finalizing the proposal to use the FY 2025 pre-floor, pre-reclassified hospital wage index data as the basis for the FY 2025 hospice wage index. The wage index applicable for FY 2025 is available on the website. The hospice wage index for FY 2025 is effective October 1, 2024, through September 30, 2025. The effective date is October 1, 2024.
- The hospice payment update includes a statutory aggregate cap that limits the overall payments per patient that may be made to a hospice annually. The final hospice cap amount for the 2025 fiscal year is $34,465.34.
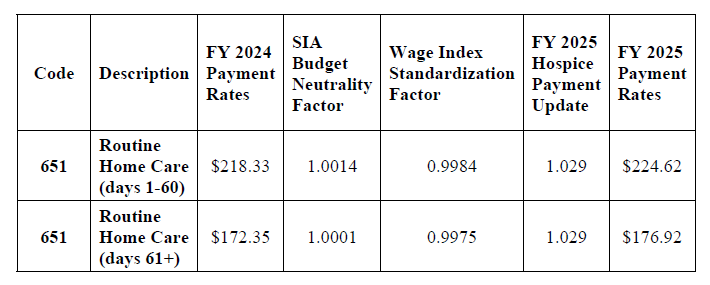
Final FY 2025 Hospice RHC Payment Rates
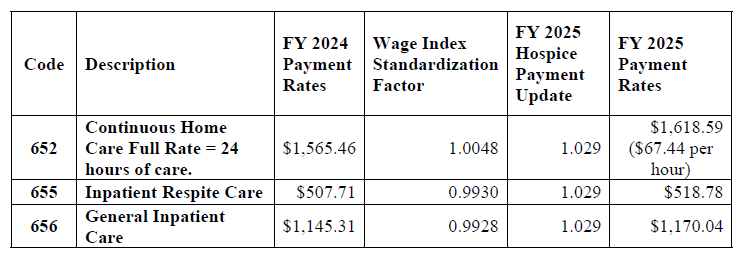
Final FY 2025 Hospice CHC, IRC, and GIP Payment Rates
Final regulatory text clarifications (see #9 for regulatory text updates)
- Discrepancy between hospice CoP regulations and payment requirements:
- CMS finalized to align the medical director CoP and the hospice payment requirements for both clarity and consistency, we are proposing technical changes to the CoPs by adding the physician member of the hospice IDG as an individual who may review the clinical information for each patient and provide written certification that it is anticipated that the patient’s life expectancy is six months or less if the illness runs its normal course.
- Confusion between the hospice election statement and the Notice of Election (NOE)
- CMS finalized to reorganize the language at § 418.24 to clearly denote the differences between the election statement and the NOE. That is, we are proposing to title § 418.24(b) as “Election Statement” and would include the title “Notice of Election” at § 418.24(e). By clearly titling this section, the requirements for the election statement and the notice of election would be distinguished from one another, mitigating any confusion between the two documents.
- Request for Information (RFI) on Payment Mechanism for High-Intensity Palliative Care Services
- CMS solicited public comments in the proposed rule on six questions focusing on the provision of high-intensity palliative care. They thanked the commenters in the final rule for their insight and thoughtful recommendations and expressed appreciation for the time and effort readers put forth in collaborating with CMS as they explore ways to improve coverage under the Medicare hospice benefit. CMS will consider all comments and recommendations received on the rule and will continue to welcome thoughts regarding these issues through our hospice policy mailbox at hospicepolicy@cms.hhs.gov.
- Changes to the Hospice Quality Reporting Program (HQRP)
- New Quality Measures
- CMS proposes to add two new process measures to HQRP.
- Timely Reassessment of Pain Impact and Timely Reassessment of Non-Pain Symptom Impact are expected to begin collection in FY 2028.
- These two measures would use the data from the HOPE assessment tool.
- These process measures would reflect whether a follow-up visit occurred within 48 hours of an initial assessment where there was an impact of moderate or severe symptoms with and without pain.
CMS is finalizing the measures with modifications from the version proposed in the proposed rule. As finalized, these QMs measure whether patients receive an in-person nursing follow-up visit within 2 calendar days of the initial assessment of moderate to severe symptoms impact. Theses (SFVs) may be performed by RNs or LPNs/LVNs.
- HOPE Assessment Tool
CMS finalizes the adoption and implementation of the Hospice Outcomes and Patient Evaluation (HOPE) patient-level data collection tool. HOPE data reporting and collection will be effective beginning on or after October 1, 2025, to support the quality measures anticipated for public reporting on or after FY 2028.
- Implementation would update § 418.312(a)(b)(1) to require hospices to complete and submit a standardized set of items for each patient to capture patient-level data, regardless of payer or patient age.
- The HOPE tool will replace the existing Hospice Item Set (HIS) structure upon implementation.
- HOPE will collect data at multiple time points across the hospice stay, including admission, the HOPE Update Visit (HUV), and discharge. Compared to the HIS (which only collected data at hospice admission and discharge), HOPE will enable CMS to gather patient-level data during their hospice stay to support quality measures.
- HOPE includes several domains that are new or expanded relative to HIS, including:
- Sociodemographic (updated)
- Diagnoses (expanded)
- Symptom Impact Assessment
- Imminent death
- RFI – CMS requested stakeholder input on potential data collection items related to four SDOHs (housing instability, food insecurity, utility, and transportation challenges) that may be relevant to the hospice setting and how they may need to be adapted to be better suited for the hospice setting.
- CMS expressed appreciation for all stakeholders’ input regarding the potential inclusion of additional SDOH items in HQRP, among other efforts to improve hospice health equity. They will consider this input on other recommended potential SDOH items in HQRP as they continue to develop and work towards the implementation of these data elements.
- CMS will provide information about upcoming provider training related to HOPE v1.0 that will be posted on the CMS HQRP website on the HQRP Announcement and Spotlight page and announced during Open Door Forums.
- The draft HOPE Guidance Manual v1.0 is available for review and the final HOPE Guidance Manual v1.0 will be available after the publication of the final rule. This guidance manual offers hospices direction on the collection and submission of hospice patient stay data to CMS to support the HQRP quality measures.
- Software developers and vendors should not wait for final technical data specifications to begin development of their own products. Software developers and vendors are encouraged to thoroughly review the draft technical data specifications and provide feedback to CMS so we may address potential issues adequately and promptly.
- CMS will conduct a call with software developers and vendors after the draft specifications are posted, during which we will respond to questions, comments, and suggestions.
- Hospice providers will need to use vendor software to submit HOPE records to CMS. As with HIS, facilities that fail to submit all required HOPE assessments to CMS for at least 90% of their patients will be subject to a 4% reduction. See the “Submission of Data Requirements” section below for additional information.
- CMS will retire the Hospice Abstraction Reporting Tool (HART) on October 1, 2025, hospices will need to select a private vendor to collect and submit HIS data, and subsequently HOPE data, to CMS.
- The final HOPE Guidance Manual v1.0 will be available on the HQRP HOPE webpage after the publication of the final rule. This guidance manual offers hospices direction on the collection and submission of hospice patient stay data to CMS to support the HQRP quality measures.
- Updated Hospice CAHPS Survey
- CMS finalized changes to the Hospice CAHPS Survey based on the results of a mode experiment conducted in 2021. CMS is finalizing implementation for April 2025 decedents, allowing hospices and vendors additional time to prepare.
- Survey vendors will be evaluated as to their readiness to administer the updated CAHPS Hospice Survey, as well as the web-mail mode.
- Training materials will be made available in early fall 2024; administration for April 2025 decedents is not slated to begin until summer 2025, allowing approximately 10 months for vendors to program and prepare materials.
- A draft of the updated survey instrument is already available for survey vendor review on the CAHPS Hospice Survey website (https://www.hospicecahpssurvey.org/globalassets/hospice-ahps4/surveyinstruments/revised_cahps-hospice-survey_for-website.pdf).
- Changes to the CAHPS survey:
- The addition of a web-mail mode (email invitation to a web survey, with mail follow-up to non-responders). The web-mail mode is optional; hospices do not need to select this mode in the first quarter in which it is available. Rather, hospices may choose to pursue this mode for any future quarter, when they and their EMR vendors are ready to provide caregiver email addresses.
- A shortened and simplified survey
- Modifications to survey administration protocols to include a prenotification letter and extended field period from 42 days to 49 days
- The addition of a new, two-item Care Preferences measure
- Revisions to the existing Hospice Team Communication measure and the existing Getting Hospice Care Training measure
- The removal of three nursing home items
- Impact of updated CAHPS survey on the Special Focus Program (SFP)
- The Hospice Special Focus Program (SFP) algorithm uses data from four measures related to caregiver experience collected by the CAHPS Hospice Survey, including Help for Pain and Symptoms, Getting Timely Help, Willingness to Recommend this Hospice, and Overall Rating of this Hospice.
- Finalized: Includes changes to the Overall Rating of this Hospice measure that are non-substantive and will not impact the SFP algorithm.
- Impact of updated CAHPS survey on CAHPS Star Ratings
- CMS proposes waiting to publicly report the new version of “Getting Hospice Care Training” until it has eight quarters of data. It anticipates that the first Care Compare refresh in which publicly reported measures scores would be updated to include the new measures would be in February 2028 (FY 2028) 2028), with scores calculated using data from Q2 2025 through Q1 2027.
- During the transition period, scores and Star Ratings would be calculated by combining scores from quarters using the current and new survey. As a result of the survey measure changes, we propose that the Family Caregiver Survey Rating Summary Star Rating will be based on seven measures rather than the current eight measures during the interim period until a full eight quarters of data are available for the “Getting Hospice Care Training” measure.
- The summary Star Rating would be based on nine measures once eight quarters of data are available for the new Care Preference and Getting Hospice Care Training measures.
- HQRP Compliance
- The compliance threshold for HOPE record submission (and acceptance) is 90%.
- The compliance threshold for CAHPS is the submission of CAHPS data by a CMS-approved vendor for four quarters in a calendar year by the specified dates.
- If these thresholds are not met, or no data is submitted, the provider will be subject to a 4% payment deduction in the coordinating APU year.
HQRP Compliance Checklist
| Annual payment update | HIS/HOPE | CAHPS |
| FY2025 | Submit at least 90 percent of all HIS records within 30 days of the event date (for example patient’s admission or discharge) for patient admissions/discharges occurring 1/1/23-12/31/23 | Ongoing monthly participation in the Hospice CAHPS survey 1/1/2023- 12/31/2023 |
| FY2026 | Submit at least 90 percent of all HIS records within 30 days of the event date (for example, a patient’s admission or discharge) for patient admissions/discharges occurring 1/1/24-12/31/24 | Ongoing monthly participation in the Hospice CAHPS survey 1/1/2024-12/31/2024 |
| FY 2027 | Submit at least 90 percent of all HIS/HOPE records within 30 days of the event date (for example, patient’s admission or discharge) for patient admissions/discharges occurring 1/1/25-12/31/25 | Ongoing monthly participation in the Hospice CAHPS survey 1/1/2025-12/31/2025 |
| FY 2028 | Submit at least 90 percent of all HIS/HOPE records within 30 days of the event or completion date (for example, patient’s admission date, HUV completion date or discharge date) for patient admissions/discharges occurring 1/1/26-12/31/26 | Ongoing monthly participation in the Hospice CAHPS survey 1/1/2026-12/31/2026 |
- Updates to Hospice federal regulatory text
| Regulation | Update |
| § 418.25 Admission to hospice care | § 418.22 Certification of terminal illness. (c)(1)(i) The medical director of the hospice, the physician designee (as defined in § 418.3), or the physician member of the hospice interdisciplinary group; and 3. Section 418.24 is amended by– a. Revising paragraphs (a) and (b)(3); b. Redesignating paragraphs (e) through (h) as paragraphs (f) through (i), respectively; and c. Adding paragraph (e). |
| § 418.24 Election of hospice care | (a) Election statement. An individual who meets the eligibility requirement of § 418.20 may file an election statement with a particular hospice. If the individual is physically or mentally incapacitated, his or her representative (as defined in § 418.3) may file the election statement. (b) * * * (3) Acknowledgement that the individual has been provided information on the hospice’s coverage responsibility and that certain Medicare services, as set forth in paragraph (g) of this section, are waived by the election. For Hospice elections beginning on or after October 1, 2020, this would include providing the individual with information indicating that services unrelated to the terminal illness and related conditions are exceptional and unusual and hospice should be providing virtually all care needed by the individual who has elected hospice. * * * * * (e) Notice of election. The hospice chosen by the eligible individual (or his or her representative) must file the Notice of Election (NOE) with its Medicare contractor within 5 calendar days after the effective date of the election statement. (1) Consequences of failure to submit a timely notice of election. When a hospice does not file the required Notice of Election for its Medicare patients within 5 calendar days after the effective date of election, Medicare will not cover and pay for days of hospice care from the effective date of election to the date of filing of the notice of election. These days are a provider liability, and the provider may not bill the beneficiary for them. (2) Exception to the consequences for filing the NOE late. CMS may waive the consequences of failure to submit a timely-filed NOE specified in paragraph (e)(1) of this section. CMS will determine if a circumstance encountered by a hospice is exceptional and qualifies for waiver of the consequence specified in paragraph (e)(1) of this section. A hospice must fully document and furnish any requested documentation to CMS for a determination of exception. An exceptional circumstance may be due to, but is not limited to, the following: (i) Fires, floods, earthquakes, or similar unusual events that inflict extensive damage to the hospice’s ability to operate. (ii) A CMS or Medicare contractor systems issue that is beyond the control of the hospice. (iii) A newly Medicare-certified hospice that is notified of that certification after the Medicare certification date, or which is awaiting its user ID from its Medicare contractor. (iv) Other situations determined by CMS to be beyond the control of the hospice. |
| § 418.25 Admission to hospice care | (a) The hospice admits a patient only on the recommendation of the medical director (or the physician designee, as defined in § 418.3) in consultation with, or with input from, the patient’s attending physician (if any). (b) In reaching a decision to certify that the patient is terminally ill, the hospice medical director (or the physician designee, as defined in § 418.3) must consider at least the following information: * * * * * 5. Section 418.102 is amended by revising the introductory paragraph, paragraph (b) introductory text, and paragraph (c) to read as follows: |
| § 418.102 Condition of participation: Medical director | The hospice must designate a physician to serve as medical director. The medical director must be a doctor of medicine or osteopathy who is an employee or is under contract with the hospice. When the medical director is not available, a physician designee as defined at § 418.3 assumes the same responsibilities and obligations as the medical director. * * * * * (b) Standard: Initial certification of terminal illness. The medical director (or physician designee, as defined in § 418.3, if the medical director is unavailable) or physician member of the IDG reviews the clinical information for each hospice patient and provides written certification that it is anticipated that the patient’s life expectancy is 6 months or less if the illness runs its normal course. The physician must consider the following when making this determination: * * * * * (c) Standard: Recertification of the terminal illness. Before each recertification period for each patient, as described in § 418.21(a), the medical director (or physician designee, as defined in § 418.3, if the medical director is unavailable) or physician member of the IDG must review the patient’s clinical information. |
View the Final Rule on the Federal Register’s Public Inspection Desk
For further information, see the CMS summary of the hospice final rule and the hospice webpage.
Questions about the content of this rule?